Introduction
The combination of age-related changes in the cardiovascular system and the increasing prevalence of cardiovascular disease at older age predispose the older individual to the development of heart failure (HF). As a result, HF is predominantly a disorder of older adults, with persons over age 75 accounting for more than 50% of the over 1 million hospitalizations for HF each year in the United States, and over 60% of all HF-related deaths.1 In addition, both the incidence and prevalence of HF are increasing, primarily due to the ageing of the population, and it is anticipated that the number of older adults with clinical HF will double over the next 25 years.
In addition to its effects on hospitalizations and mortality, HF is an important cause of chronic disability in older adults, and the functional limitations imposed by HF are often a key factor contributing to entry into a long-term care facility. HF is also one of the most common comorbid conditions in hospitalized older adults who develop delirium, and HF interacts detrimentally with every major geriatric syndrome. Thus, the societal burden attributable to HF in the ageing population is extremely high, as a consequence of which HF is one of the most costly medical illnesses in the United States today, with estimated annual expenditures in excess of $39 billion, representing approximately 5% of the total healthcare budget.1
Pathophysiology
Cardiovascular Ageing
As discussed in Chapter 35, normal ageing is associated with extensive changes in cardiovascular structure and function. Taken together, these changes result in a marked reduction in cardiovascular reserve, so that older adults are less able to maintain normal cardiac output and intracardiac pressures in response to stress, whether that stress is physiologic (e.g. exercise) or pathologic (e.g. ischaemia, anaemia, infection). Figure 41.1 illustrates the striking effect of normal ageing on maximum oxygen consumption (VO2 max) in healthy men and women carefully screened to exclude occult cardiovascular disease. Note that the decline in VO2 max with age is not simply linear, but that it actually accelerates after age 60. Moreover, normal octogenarians often have VO2 max levels of less than 20 ml O2min−1kg−1, which are similar to those typically observed in middle-aged persons with moderate HF (New York Heart Association class II). Given the normal decline in cardiovascular reserve with increasing age, it is not difficult to understand why an 85-year-old who suffers an acute myocardial infarction (MI) is substantially more prone to develop HF and cardiogenic shock than a 65-year-old who suffers an MI of equivalent size. Similarly, older patients are more likely to develop HF in response to numerous other cardiac and non-cardiac stressors, such as atrial fibrillation (AF), pneumonia, intravenous fluid administration, or any type of major surgery.
Figure 41.1 Age and VO2max in healthy subjects: the Baltimore Longitudinal Study on Aging. Reproduced from Fleg JL et al. Longitudinal decline of aerobic capacity accelerates with age. Circulation 2000;102(Suppl II):II–602 [abstract], by permission of Lippincott Williams & Wilkins.
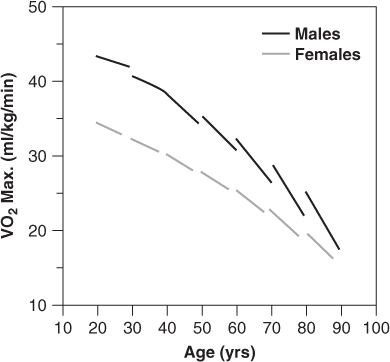
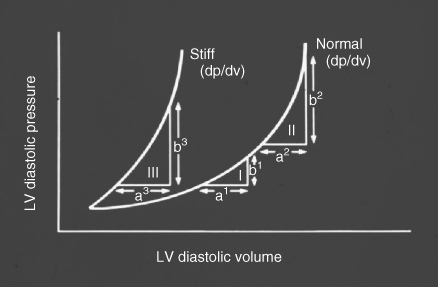
Table 41.1 summarizes the principal effects of normal ageing on cardiovascular structure and function.2, 3 Increased vascular stiffness contributes to the progressive rise in systolic blood pressure at older age, and increases impedance to left ventricular (LV) ejection (afterload). Impaired LV relaxation during early diastole (an active, energy-requiring process) and increased myocardial stiffness markedly alter the pattern of LV diastolic filling (preload) and result in a shift in the LV pressure-volume relationship upwards and to the left (Figure 41.2). These changes result in an increased reliance on atrial contraction (the ‘atrial kick’) to optimize LV filling, and predispose the older individual to the development of HF with preserved LV ejection fraction (HFPEF) and AF. Diminished responsiveness to β-adrenergic stimulation attenuates the heart rate response to stress and also reduces peak contractility, both of which are dependent on activation of the cardiac β1-receptors. In addition, peripheral vasodilation is impaired due to reduced responsiveness of arteriolar β2-receptors, further increasing afterload and limiting skeletal muscle blood flow during exercise. In healthy older adults, mitochondria in the cardiac myocytes are capable of generating sufficient ATP to meet the resting energy needs of the myocardium, but they have reduced capacity to increase ATP production in response to stress, thus further limiting peak cardiac performance. There is also a reduction in the number of functioning sinus node pacemaker cells with age, giving rise to the ‘sick sinus syndrome’ and contributing to chronotropic incompetence; that is, the inability to increase heart rate commensurate with demands. Finally, age-related endothelial dysfunction and vasomotor dysregulation, while not affecting cardiac performance directly, limit peak coronary blood flow and contribute to the development and progression of atherosclerosis and coronary artery disease (CAD). In summary, normal cardiovascular ageing exerts deleterious effects on all four of the major determinants of cardiac output—heart rate, contractility, preload, and afterload—thereby greatly reducing peak cardiac performance and cardiovascular reserve. In addition, cardiovascular ageing fosters the development of systolic hypertension and CAD, the two leading causes of HF in older adults.1
Table 41.1 Principal effects of ageing on cardiovascular structure and function.
|
ATP, adenosine triphosphate
Figure 41.2 Effect of age on the left ventricular (LV) pressure-volume relationship. Note that there is a shift to the left, such that small increases in left ventricular volume are associated with greater increases in left ventricular pressure compared to younger persons. Adapted from Gaasch WH et al., Am J Cardiol 1976;38:645–53.
Other Organ Systems
Age-associated changes in other organ systems also contribute to the predilection of older adults to develop HF, and may affect the clinical features and response to therapy (Table 41.2). Renal function declines with age, and older adults are less able to excrete a salt and water load. Pulmonary reserve also declines with age, with decreased vital capacity and increased ventilation-perfusion mismatching resulting in more severe hypoxaemia in the setting of superimposed HF. The central nervous system is less able to maintain cerebral perfusion in response to decreased cardiac output due to impaired autoregulatory capacity, thus increasing the propensity of older HF patients to develop impaired cognition or overt delirium. Thirst is also impaired in older adults, increasing the risk of diuretic-induced dehydration. Sarcopenia, a hallmark of ageing, contributes to impaired exercise tolerance and diminished aerobic capacity in older HF patients. Finally, age-related alterations in the alimentary tract, liver, and kidneys result in substantial changes in the absorption, metabolism, and excretion of virtually all medications.
Table 41.2 Effects of ageing on other organ systems.
| Kidneys Decline in glomerular filtration rate (GFR), ∼8 cm3 min−1per decade Impaired water and electrolyte homeostasis Reduced plasma renin and aldosterone activity Impaired elimination of renally excreted drugs |
| Lungs Loss of elastic recoil Increased ventilation-perfusion (V/Q) mismatching Reduced vital capacity and minute ventilation |
| Nervous system Diminished reflex responsiveness, esp. baroreceptors Reduced central nervous system autoregulatory capacity Impaired thirst mechanism |
| Musculoskeletal system Loss of muscle mass and strength (sarcopenia) Loss of bone mass, esp. in women (osteopenia) |
| Altered pharmacokinetics and pharmacodynamics of most drugs |
Clinical Features
Symptoms and Signs
Exertional dyspnoea, orthopnea, lower extremity swelling, and impaired exercise tolerance are the cardinal symptoms of HF at both younger and older age. However, with increasing age, which is often accompanied by a progressively more sedentary lifestyle, exertional symptoms become less prominent. Conversely, atypical symptoms, such as confusion, somnolence, irritability, fatigue, anorexia, or diminished activity level, become increasingly more common manifestations of HF, especially after age 80.
Physical signs of HF include elevated jugular venous pressure, hepatojugular reflux, an S3 gallop, pulmonary rales, hepatomegaly, and dependent oedema. With the exception of rales each of these features occurs less commonly in older HF patients, in part because of the increasing prevalence of HFPEF, in which signs of right HF are a late manifestation and a third heart sound is typically absent. On the other hand, behavioural changes and altered cognition, which may range from subtle abnormalities to overt delirium, frequently accompany HF at elderly age, particularly among institutionalized or hospitalized patients.
Diagnosis
Accurate diagnosis of the HF syndrome at older age is confounded in part by the increasing prevalence of atypical symptoms and signs. In addition, exertional symptoms may be attributable to non-cardiac causes, such as pulmonary disease, anaemia, depression, physical deconditioning, or ageing itself. Likewise, peripheral oedema may be due to venous insufficiency, hepatic or renal disease, or medication side effects (e.g. calcium-channel blockers), and pulmonary crepitus may be due to atelectasis or chronic lung disease. Despite these limitations, careful clinical assessment for the presence of multiple symptoms and signs should lead to the correct diagnosis in most cases.
Chest radiography is indicated when HF is suspected, and it remains the most useful diagnostic test for determining the presence of pulmonary congestion. However, chronic lung disease, altered chest geometry (e.g. due to kyphosis), or poor inspiratory effort may confound interpretation of the chest radiograph in elderly individuals.
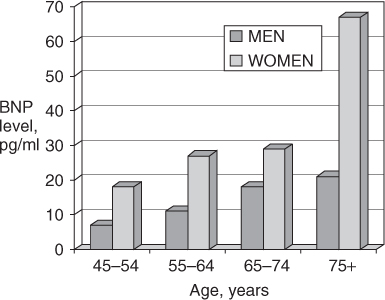
Plasma B-type natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) levels have been shown to be a valuable aid in distinguishing dyspnoea due to HF from that related to other causes, such as pulmonary disorders. BNP and NT-proBNP levels tend to be elevated in both systolic HF and HFPEF, and they also correlate with response to therapy and prognosis. However, levels of these peptides also increase with age in healthy individuals without HF, particularly women (Figure 41.3), and as a result, the specificity and predictive accuracy of elevated levels decline with age.4 Nonetheless, in cases of diagnostic uncertainty, a low or normal BNP or NT-proBNP level effectively excludes acute HF, whereas markedly elevated levels provide strong evidence in support of the diagnosis.
Figure 41.3 Mean B-type natriuretic peptide (BNP) levels in healthy volunteers according to age and gender. Adapted from Redfield MM et al.4
Proper management of HF is critically dependent on establishing the pathophysiology of LV dysfunction (i.e. systolic vs. diastolic), determining the primary and any secondary aetiologies (Table 41.3), and identifying potentially treatable precipitating or contributory factors (Table 41.4). Differentiating systolic from diastolic dysfunction requires an assessment of LV ejection fraction by echocardiography, radionuclide ventriculography, magnetic resonance imaging, or contrast angiography. Among these, echocardiography is the most widely used and clinically useful non-invasive test for evaluating systolic and diastolic function. In addition, echocardiography provides important information about LV chamber size and wall thickness, atrial size, right ventricular function, the presence and severity of valvular lesions, and pericardial disorders. For these reasons, echocardiography is recommended for all patients with newly diagnosed HF or unexplained disease progression.5
Table 41.3 Common aetiologies of heart failure in older adults.
| Coronary artery disease Acute myocardial infarction Chronic ischaemic cardiomyopathy |
| Hypertensive heart disease Hypertensive hypertrophic cardiomyopathy |
| Valvular heart disease Aortic stenosis or insufficiency Mitral stenosis or insufficiency Prosthetic valve malfunction Infective endocarditis |
| Cardiomyopathy Dilated (non-ischaemic) Alcohol Chemotherapeutic agents Inflammatory myocarditis Idiopathic Hypertrophic Obstructive Non-obstructive Restrictive (esp. amyloid) |
| Pericardial disease Constrictive pericarditis |
| High output syndromes Chronic anaemia Thiamine deficiency Hyperthyroidism Arteriovenous shunting |
| Age-related diastolic dysfunction |
Table 41.4 Common precipitants of heart failure in older adults.
| Myocardial ischaemia or infarction |
| Uncontrolled hypertension |
| Dietary sodium excess |
| Medication non-adherence |
| Excess fluid intake Self-induced Iatrogenic |
| Arrhythmias Supraventricular, esp. atrial fibrillation Ventricular Bradycardia, esp. sick sinus syndrome |
| Associated medical conditions Fever Infections, esp. pneumonia or sepsis Hyperthyroidism or hypothyroidism Anaemia Renal insufficiency Thiamine deficiency Pulmonary embolism Hypoxemia due to chronic lung disease |
| Drugs and medications Alcohol Beta-adrenergic blockers (incl. ophthalmologicals) Calcium-channel blockers Anti-arrhythmic agents Non-steroidal anti-inflammatory drugs Glucocorticoids Mineralocorticoids Estrogen preparations Anti-hypertensive agents (e.g. clonidine, minoxodil) |
Other diagnostic studies that may be indicated in selected patients include an assessment of thyroid function (especially in the presence of AF), an exercise or pharmacologic stress test to evaluate for the presence and severity of ischaemia, and cardiac catheterization if revascularization or other corrective procedure (e.g. valve repair or replacement) is being contemplated.
Aetiology and Precipitating Factors
Systemic hypertension and CAD account for 70–80% of HF cases at older age.1 Hypertension is the most common aetiology in older women, particularly those with preserved ejection fraction. In older men, HF is more often attributable to CAD. Other common aetiologies include valvular heart disease (especially aortic stenosis and mitral regurgitation) and non-ischaemic cardiomyopathy (Table 41.3). Importantly, HF in the elderly is frequently multifactorial, and it is thus essential to identify all potentially treatable causes.
In addition to determining aetiology, it is important to identify factors precipitating or contributing to HF exacerbations (Table 41.4). Non-adherence to medications and dietary restrictions is the most common cause of worsening HF, and patients should be closely questioned about their dietary and medication habits. Other common factors contributing to increased symptoms include ischaemia, volume overload due to excess fluid intake (self-inflicted or iatrogenic), tachyarrhythmias (especially AF or flutter), intercurrent infections, anaemia, thyroid disease, and various medications or toxins (e.g. alcohol).
Comorbidity
A hallmark of ageing is the increasing prevalence of multiple comorbid conditions, many of which impact directly or indirectly on the diagnosis, clinical course, treatment, and prognosis of HF in the elderly (Table 41.5). As noted previously, renal function declines with age, and octogenarians often have creatinine clearances of <60 cm3 min−1 (i.e. stage III chronic kidney disease), despite ‘normal’ serum creatinine levels and in the absence of underlying renal disease. Older patients are also less able to excrete excess sodium and water, and this deficiency may contribute to volume overload. Diuretics tend to be less effective in the elderly, whereas diuretic-induced electrolyte disorders are more common, in part due to reduced capacity of the kidneys to preserve electrolyte homeostasis. Conversely, diuretics, ACE inhibitors, and angiotensin receptor blockers (ARBs) can all contribute to worsening renal function, and older patients are at increased risk for this complication.
Table 41.5 Common comorbidities in older patients.
| Condition | Implications |
| Renal dysfunction | Exacerbated by diuretics, |
| ACE inhibitors, ARBs | |
| Anaemia | Worsens symptoms and prognosis |
| Chronic lung disease | Contributes to uncertainty about |
| diagnosis and volume status | |
| Cognitive dysfunction | Interferes with dietary, medication and activity adherence |
| Depression, | Worsens prognosis, |
| social isolation | interferes with adherence |
| Postural hypotension, falls | Exacerbated by vasodilators, diuretics, beta-blockers |
| Arthritis | NSAIDs worsen heart failure, antagonize heart failure medications |
| Urinary incontinence | Aggravated by diuretics, ACE inhibitors (cough) |
| Sarcopenia, osteoporosis | Contribute to impaired exercise tolerance |
| Sensory deprivation | Interferes with adherence |
| Nutritional disorders | Exacerbated by dietary restrictions |
| Polypharmacy | Reduced adherence, increased drug interactions |
| Frailty | Exacerbated by hospitalization; increased fall risk |
ACE, angiotensin-converting enzyme; ARBs, angiotensin-receptor blockers; NSAIDs, non-steroidal anti-inflammatory drugs
Older HF patients are at increased risk for anaemia due to comorbid chronic illnesses (renal disease, occult malignancy), inadequate dietary intake of key nutrients (iron, folate, B12), and use of medications associated with gastrointestinal blood loss (aspirin, warfarin, non-steroidal anti-inflammatory drugs). Anaemia contributes to impaired tissue oxygen deliver and impaired exercise tolerance, and may exacerbate myocardial ischaemia in patients with underlying CAD. Anaemia has also been shown to be an independent predictor of adverse clinical outcomes in patients with HF.
Normal ageing is associated with a decline in maximum voluntary ventilation and an increase in ventilation-perfusion mismatching. In addition, chronic obstructive and restrictive lung diseases further impair pulmonary function in many older adults. Diminished pulmonary function, in turn, contributes to increased dyspnoea and exercise intolerance in older patients with HF, as the lungs are unable to compensate for impaired cardiac performance. In addition, the presence of chronic lung disease often leads to diagnostic uncertainty (is the patient’s dyspnoea due to HF, pulmonary disease, or a combination of both?), in part by confounding interpretation of the physical examination and chest radiograph.
Cognitive dysfunction interferes with the patient’s ability to participate fully in self-care behaviours, such as weight monitoring and adherence to dietary restrictions and prescribed medications. In more severe cases, cognitive impairment substantially limits the patient’s ability to provide a reliable medical history, and may prevent recognition of new or worsening symptoms. Patients with cognitive dysfunction are also at increased risk for developing delirium, which may complicate hospital management and predispose to serious adverse events (e.g. falls, aspiration, infections).
Up to 20% of elderly HF patients have clinically significant depression, which is often unrecognized. Social isolation, primarily due to the death of one’s spouse, also occurs with increasing frequency at elderly age. These conditions have both been associated with adverse outcomes in elderly HF patients, including increased mortality and hospitalization rates, in part due to reduced adherence to prescribed medications and other recommended behaviours. In addition, depression has been linked with increased adrenergic tone and ventricular arrhythmias in patients with cardiovascular disease, both of which confer an increased mortality risk in HF patients.
Increased vascular stiffness and impaired baroreflex responsiveness predispose older adults to the development of postural hypotension, while age-related alterations in sinus node function increase the risk of bradyarrhythmias (sick sinus syndrome). In addition, balance and proprioception decline with age. Taken together, these factors greatly increase the risk of falls in older adults. Standard HF therapies, including diuretics, vasodilators, and beta-blockers, all have the potential to further increase the risk of falls and associated morbidity.
Arthritis is the leading cause of chronic disability in older adults, and is widely treated with non-steroidal anti-inflammatory drugs (NSAIDs) available both by prescription and over-the-counter. These agents enhance renal sodium and water reabsorption and have been associated with a significant increase in the risk of hospitalization for HF, even among patients with no prior HF history.6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








