Kent A. Sepkowitz The potential for bloodborne transmission of hepatitis B first was noted in 1885, when Lurman described jaundice in factory workers who had received smallpox vaccine prepared from “human lymph.”1 More reports appeared in the subsequent decades as use of vaccines derived from human serum became more common.2 In addition, more frequent use of phlebotomy equipment,3 insulin therapy,4 and intramuscular injection of antibiotics all led to small outbreaks of jaundice, which were ascribed to a transmissible “icterogenic” agent. By the late 1940s, studies to clarify the modes of transmission were undertaken. Central to these was the use of human volunteers, who were given putatively infectious material intradermally, intranasally, or by ingestion of feces, and then observed for development of jaundice.2,5–7 From these landmark reports arose our current understanding of the basic principles of transmission of infectious hepatitis (hepatitis A) and serum hepatitis (hepatitis B). The first report of occupational disease in health care workers (HCWs) was provided by Leibowitz and colleagues,8 who described jaundice in a blood bank nurse with numerous needle pricks on her hands and fingers. A spate of similar reports followed, describing occupationally acquired hepatitis among nurses, blood bank workers, phlebotomists, house staff, and others.9,10 Soon, the workers’ compensation boards of certain states ruled that viral hepatitis was a compensable occupational hazard. Improved understanding of routes of transmission, more comprehensive and rigorous infection control including needle disposal, and, for hepatitis B, vaccination of workers at risk have helped to decrease, but not eliminate, this occupational risk. A corollary risk, that of transmission of infection from infected HCWs, particularly surgeons, to nonimmune patients, has been described for hepatitis B11,12 and hepatitis C.13 In recent years, observed needlesticks have less commonly been the cause of health care–associated hepatitis. Rather, most transmissions have resulted from other health care exposures, such as those that occur from improperly used medical equipment or devices, often involving injection of patients.14,15 These include endoscopes, injection syringes, fingerstick devices, multiuse vials, and intravenous therapies. Glucose monitoring via needlesticks, particularly in assisted living facilities, is frequently described.16 Diversion of medication, whereby a practitioner personally administers an intravenous narcotic and then re-uses the remainder of the vial of medication on a patient or patients, is also increasingly recognized as a source of transmission.17 The Centers for Disease Control and Prevention (CDC) maintains a web-based inventory of reported hepatitis B and C outbreaks identified since 2008.18 Per the report, 31 outbreaks affecting more than 150 people have been investigated between 2008 and 2011. Of these, the vast majority have occurred outside the hospital, particularly in various types of long-term care facilities. This website updates their previous review of outbreaks spanning 1992 to 2008.19 The CDC does not recommend routine vaccination of HCWs to protect against hepatitis A.20 However, rare nosocomial outbreaks have been reported from pediatric or neonatal intensive care units.21–23 In addition, a few studies have demonstrated transmission to HCWs and other patients from hospitalized adults with24,25 and without26 diarrhea. Reduction of transmission by administration of intramuscular immune globulin to contacts has been used effectively for many years (Table 305-1).27 Broad-scale vaccination may also help interrupt the spread of infection. TABLE 305-1 Nosocomial Hepatitis: Transmission Rates and Interventions * Rate of transmission from outbreak or needlestick exposure. ACIP, Advisory Committee on Immunization Practices; HBeAg, hepatitis B e antigen; HBIG, hepatitis B immune globulin; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCWs, health care workers; PEG IFN, pegylated interferon. Outbreaks of hepatitis E have occurred in resource-constrained countries, but nosocomial transmission has not been described in the West. Increasing reports of liver transplant patients with chronic hepatitis E suggest that the infection is not transmitted at transplant but rather is latent among certain patients with exposure to pig and swine.28 In Pakistan, an outbreak of hepatitis E affected up to 18 people (7 confirmed and 11 possible cases). Assessment of the outbreak suggested that spread occurred due to improper sharing of intravenous equipment sets among patients.29 Hepatitis B was the first bloodborne disease recognized to pose an occupational hazard.8–10 An early review found a preponderance of cases among pathologists, laboratory workers, and blood bank workers.9 Vaccine to prevent hepatitis B virus (HBV) infection became available in the United States in 1982. Introduction of the currently used recombinant HBV vaccine improved vaccine acceptance. In 1987, concern about occupational acquisition of the human immunodeficiency virus (HIV) led the U.S. Department of Labor, in conjunction with the Department of Health and Human Services, to recommend Universal Precautions to protect against exposure to body fluids.30 Four years later, the Occupational Safety and Health Administration published the federal Bloodborne Pathogens Standard, which went into effect in early 1992.31 This document mandated that HCWs with potential exposure to blood or other potentially infectious materials should either be offered the hepatitis B vaccine series free of charge, demonstrate immunity to hepatitis B, or formally decline vaccination. Compliance with this recommendation has resulted in a 95% reduction of occupationally acquired hepatitis B,32,33 although rare cases continue to occur. Before routine vaccination, dentists, physicians, nurses, and laboratory and dialysis staff were among the HCW groups with the highest risk.9,10,34–37 The risk of transmission from a single needlestick exposure varies according to the hepatitis B e antigen (HBeAg) status of the source case. It ranges from 1% to 6% for HBeAg-negative blood to 22% to 31% for HBeAg-positive blood (see Table 305-1).38 In addition, transmission from an HBsAg-negative patient may occur. Over the past several decades, dozens of episodes involving HCW-to-patient transmission of hepatitis B have been described, resulting in hundreds of secondary cases (range, 1 to 55 secondary cases per source case).11,12,39–43 In a series of 10 clusters reported from the United Kingdom, the transmission rate ranged from 0.3% to 9%.12,42 At least 42 of the 47 HCWs involved were dentists or surgeons. No cases of transmission from dentists to others have been reported since 1987, suggesting the effectiveness of Universal Precautions,42 although lack of case clustering may prevent recognition of transmission.43 In response to these concerns, in 1991, the CDC promulgated recommendations for preventing transmission of HIV and HBV to patients during exposure-prone invasive procedures, which were modified by non-CDC experts in 2012.44,45 These and other guidelines addressing infected HCWs have been sought to balance patient safety against a worker’s right to privacy.45 Most U.S. and European guidelines now recommend that workers performing exposure-prone procedures maintain a low HBV DNA level, usually less than 1000 IU/mL.45 In a classic outbreak from the 1990s, 19 (13%) of 144 susceptible patients operated on by an HBeAg-positive thoracic surgery resident developed acute hepatitis B infection despite appropriate infection control.11 Thirteen available isolates, including the surgeon’s, were identical when compared by molecular analysis. Examination of the resident’s surgical technique suggested that small cuts in his fingers, sustained by tying sutures, resulted in entry of his blood into patients’ wounds. Ironically, the surgeon had declined hepatitis B vaccine 2 years earlier and then had become infected as the result of an occupational exposure. In most but not all of the reported instances, the source worker was HBeAg positive. However, in one series, four HBeAg-negative surgeons each transmitted disease.12 The cases occurred in England, where restriction of HBeAg-positive surgeons (principally cardiothoracic, gynecologic, orthopedic, and abdominal) is strictly enforced. Confirmation of transmission was aided by HBV DNA sequencing of both the putative source and secondary cases. Recently, hepatitis B transmission occurred in at least 2 of 232 exposed, tested patients in the United States.46 The source was an orthopedic surgeon with previously undetected HBV and an HBV DNA level of almost 18 million IU/mL. Of note, he had twice received a three-dose HBV vaccination series but had not been evaluated for chronic HBV infection, despite failing to respond to the vaccine. Widespread transmission from a single patient to several HCWs is rare. In one instance, a patient in the preclinical window period for hepatitis B sustained severe trauma and underwent several surgeries.47 At least four HCWs, including nurses and physicians, developed acute hepatitis temporally consistent with transmission from this patient. As with worker-to-patient transmission, clustered cases lead to the identification of transmission more readily than individual transmissions. Therefore, other instances of transmission may go undetected and unreported. For many years, dialysis patients and staff were at high risk for occupational acquisition of hepatitis B, given their high frequency of sharp injury or mucocutaneous exposure (5 instances per 10,000 dialysis procedures48), the high titers of HBV in blood (≥109/mL), and the ability of HBV to survive well in the environment.49 However, with segregation of patients by room, staff, and machine according to surface antigen (HBsAg) status; institution of active vaccination programs; monthly serologic testing of susceptible patients; and attention to disinfection, equipment, and cleaning procedures, this rate has decreased sharply.37,49,50 When rates were compared from a classic study conducted before the availability of vaccine,37,50 the incidence of new HBsAg among patients was found to have decreased from 3% to 0.05%, and the prevalence from 7.8% to 0.9%. During the same period, vaccination coverage of staff increased to 88% and HCW HBV incidence decreased from 2.6% to less than 0.5%. Despite these gains, transmission continues in centers that fail to identify HBV-infected patients, share staff and equipment, or fail to vaccinate susceptible patients.49 One survey found that the incidence of HBV among dialysis patients was higher in centers where injectable medications were prepared on a medication cart or area, rather than in a dedicated medication room.37 In some countries, nosocomial transmission continues to account for a substantial proportion of overall hepatitis B cases.51,52 Increasingly, transmission related to spring-loaded finger-stick devices,53 endoscopy equipment, multidose medication vials, diabetic care, and jet injections has been reported,14,18,19,54,55 especially in long-term care.16,18,19,56 This group of exposures now account for up to 37% of all healthcare-associated acquisition of HBV among older adults in the United States.54 In addition, patients (so-called medical tourists) who for financial or other reasons receive care in countries with a high prevalence of HBV may unknowingly invite the additional risk of virus acquisition.57 Two patients cared for by the same oral surgeon each developed genotypically similar hepatitis B; the exact mode of transmission is unknown.58 Management in exposed or susceptible workers has been well summarized elsewhere20,38,59 (see Table 305-1 and Chapter 146). Treatment should be given within 24 hours after exposure. Intramuscular hepatitis B immune globulin (HBIG) was the original intervention for postexposure prophylaxis.60 It is still used, in conjunction with initiation of a vaccine series, for management after exposure in unvaccinated HCWs and vaccine nonresponders. Vaccine nonresponders should receive a second dose 1 month later (HBIG dose, 0.06 mL/kg).20 Vaccine and HBIG may be given at the same time but should be administered with separate needles and syringes and at different anatomic sites. Plain immune globulin does not contain sufficient titers of HBIG and should not be given.20 The role of antiviral agents such as lamivudine, entecavir, and tenofovir in the management of nosocomial exposure has not been determined. The durability of hepatitis B vaccine–induced immunity is not known.61 Vaccine-induced antibody predictably wanes in many initial responders. However, in longitudinal reports, persons with waning antibody (to <10 mIU/mL) have not developed clinical hepatitis.62 Rather, those newly infected typically develop core antibody (HBcAb) with subclinical disease. Therefore, the CDC does not recommend routine revaccination of HCWs or patients, except for dialysis patients. Dialysis patients should have their antibody level determined annually and be revaccinated when the level drops to less than 10 mIU/mL.63 Because of the cost, determination of antibody to HBV before vaccination is not recommended.20 Postvaccination testing should be routinely provided to all HCWs with an anticipated risk of occupational exposure. Knowledge of serostatus assists management of subsequent exposures.20,37 For vaccine responders, no postexposure intervention is required, regardless of the HBsAg or HBeAg status of the source. Vaccinated HCWs with an unknown response to vaccine should have their antibody titer checked immediately after exposure and be treated according to the result (see Table 305-1). Vaccine is generally well tolerated. The best long-term management for the hepatitis B vaccine nonresponder is not known. This is of particular importance because up to 10% of vaccinated persons do not seroconvert.64 All nonresponders must be tested for evidence of HBsAg. Risk factors for a suboptimal response include cigarette smoking, increasing age, obesity, and, in some series, male sex.64 Persons who do not seroconvert after the initial three-dose series should be evaluated for HBsAg carrier state and, if negative, receive a second three-dose series.20 Among those receiving a second course, up to half seroconvert. A third series for nonresponders is not recommended. Many studies have examined strategies to increase vaccine response rates, including intradermal vaccination,65 but none have been approved.
Health Care–Acquired Hepatitis
Historical Background
Current Status
Fecal-Oral Transmission
Hepatitis A
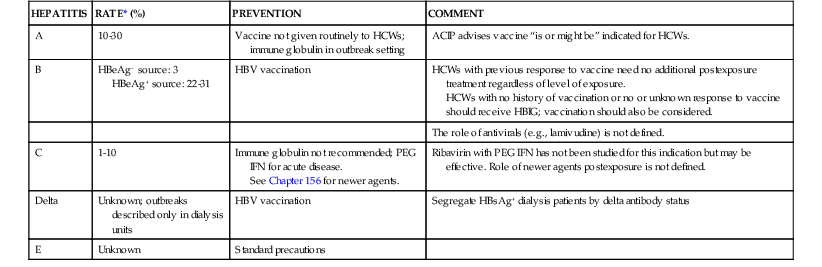
HEPATITIS
RATE* (%)
PREVENTION
COMMENT
A
10-30
Vaccine not given routinely to HCWs; immune globulin in outbreak setting
ACIP advises vaccine “is or might be” indicated for HCWs.
B
HBeAg− source: 3
HBeAg+ source: 22-31
HBV vaccination
HCWs with previous response to vaccine need no additional postexposure treatment regardless of level of exposure.
HCWs with no history of vaccination or no or unknown response to vaccine should receive HBIG; vaccination should also be considered.
The role of antivirals (e.g., lamivudine) is not defined.
C
1-10
Immune globulin not recommended; PEG IFN for acute disease.
See Chapter 156 for newer agents.
Ribavirin with PEG IFN has not been studied for this indication but may be effective. Role of newer agents postexposure is not defined.
Delta
Unknown; outbreaks described only in dialysis units
HBV vaccination
Segregate HBsAg+ dialysis patients by delta antibody status
E
Unknown
Standard precautions
Hepatitis E
Bloodborne Transmission
Hepatitis B
Epidemiology
Incidence after Exposure
Reported Transmissions
Worker-to-Patient Transmission.
Patient-to-Worker Transmission.
Dialysis Setting.
Other Nosocomial Transmissions.
Interventions and Management
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Health Care–Acquired Hepatitis
305