Head and Neck Malignancies
HEAD & NECK SQUAMOUS CELL CANCER
Alexander N. Shoushtari
Shrujal S. Baxi
David G. Pfister
Epidemiology
H&N CA: US ˜50K cases/y, 11K D/y. >90% = H&N squamous cell ca (SCCHN). M:F 3:1
Genetic syndromes: Overall, rare; Fanconi most common. Others: Lynch-2, Bloom, Li-Fraumeni, Xeroderma Pigmentosa
Special populations: Nasopharyngeal endemic in southern China and Hong Kong
Anatomic Divisions of H&N Cancer
Oral cavity: Buccal mucosa, alveolar ridges, floor of mouth, hard palate, tongue (ant 2/3)
Oropharynx: Tongue (base), tonsils, soft palate, post pharyngeal wall to level of hyoid
Nasopharynx: Superior to soft palate
Hypopharynx: Hyoid bone to cricoid cartilage
Larynx: Divided into supraglottic, glottic; subglottic (rare)
Molecular Pathogenesis
p16 inactivation: Frequent alteration in SCCHN
EGFR: Alterations in >90% of all SCCHN; overexpression correlates w/poorer prognosis (JNCI 1998;90:824)
CCND1 overexpression affects: ↑ cell cycle progression
HPV: E6, E7 viral protein → inhibit p53, Rb, other tumor suppressors
EBV: → LMP1, other viral proteins → ↑ cell replication; a/w nasopharyngeal carcinoma (esp. endemic subtype)
Pathology
Precursor lesions: Hyperplasia → Dysplasia → Carcinoma in situ → Invasive CA Leukoplakia – fixed white plaques; hyperparakeratosis w/hyperplasia Erythroplakia – red patches; often a/w epithelial dysplasia
Key pathologic findings
1° tumor – size, differentiation, depth of invasion, LVI or PNI, precursor lesions, surgical margins
Nodes – laterality, size, extracapsular extension
Molecular studies
HPV-Associated SCCHN
Initial Workup of SCCHN
Physical – Detailed H&N exam w/mirror exam or direct visualization. Consider triple endoscopy if unknown 1° or diffuse mucosal abnormalities
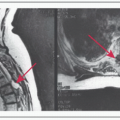
Imaging – CT/MRI of neck, chest imaging = CXR at minimum. Consider CT chest or PET/CT if N2 or N3 disease
Bx – after imaging if possible to avoid false (+), esp PET/CT; FNA of involved neck node well-tolerated, convenient; tissue HPV testing if oropharynx
Multidisciplinary care – rad onc, surgery, med onc; nutrition (PEG if malnutrition at baseline), dental, speech path, smoking/EtOH cessation
Staging (TNM)
Prognosis
HPV-associated more favorable than nonHPV; EGFR overexpression → worse
| ||||||||||||||||||||||||||||||||||||
Treatment – Curative Intent
Surgery & RT alone (early stage) or together (more advanced stage) are mainstays; chemotherapy has ↑ role in latter
Surgical margins – clear = 5 mm or more; close margins <5 mm; (+) = CIS or invasive at margin; close/(+) may dictate changes to RT or chemo plan. No OS benefit to adjuvant chemo alone (MACH-NC, Radiother Oncol 2009;92:4)
RT – when surgery is not technically feasible or undesirable (eg, larynx preservation). SEs of RT – both short and long term- fatigue, xerostomia, 2° malignancies (sarcomas). EBRT standard 66-70 Gy, 2 Gy fractions to 1° tumor and/or high-risk LNs. “Hyperfractionation” = potential higher cumulative doses in smaller fractions. Increasingly, IMRT used to minimize xerostomia (Lancet Oncol 2011;12:127) and optimize targeting but longer planning, more expertise required.
Role of Chemoradiation (CRT)
Standard is concurrent CRT; used as alternative to surgery for organ preservation or improve outcomes for unresectable or high-risk, resected disease
Larynx preservation improved w/concurrent cisplatin + RT (RTOG 91-11, JCO 2013;epub)
Adjuvant cisplatin added during post-op RT ↑ DFS (NEJM 2004;350:1937) & OS (NEJM 2004;350:1945) if (+) margins or extracapsular extension in LN (Head Neck 2005;27:843)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree