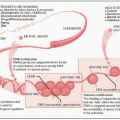
Direct effects (minor for x-rays): DNA molecules themselves are the direct targets of ionization, which ultimately trigger a chemical or biologic change
Indirect effects (major for x-rays): Radiation ionizes other molecules in the cell (most frequently water) to produce free radicals that diffuse to & cause damage to DNA
Double strand breaks (DSBs) in DNA are thought to be the critical determinant of cellular response, although many other types of molecular damage are also induced
Mitotic death: Cell death due to chromosome missegregation during mitosis
Apoptosis: Programmed cell death, in this case induced by IR
Alternative mechanisms of IR-induced lethality include senescence & autophagy
Cells that undergo apoptotic death in response to IR (ie, lymphoid cells, acinar cells of the salivary glands) are typically more radiosensitive
Correlation between rate of death & the induction of putative “lethal” chromosomal aberrations (eg, dicentrics, rings, etc.)
Cells vary in radiosensitivity across the cell cycle: Generally more sensitive in M & G2 phases & most resistant in late S phase
Oxygen enhances DNA damage induced by free radicals, thereby facilitating the indirect action of IR
Biologically equivalent dose can vary by a factor of 2-3 depending upon the presence or absence of oxygen (referred to as the oxygen enhancement ratio)
Repair of sublethal damage
Reassortment of cells w/in the cell cycle
Repopulation of cells during the course of radiotherapy
Reoxygenation of hypoxic cells
Radioresistant histologies (eg, melanoma, renal cell carcinoma, etc.) may not respond effectively to conventionally fractionated radiation doses (ie, 1.8-2 Gy)
Image-guided intensity-modulated RT (IMRT) has enabled radiation dose escalation, w/improved anatomical targeting of radiotherapy, & relative sparing of nearby nl tissues
Large radiation doses (ie, >8 Gy) may be a/w additional mechanisms of cancer cell death, including effects on the tumor-associated stroma (eg, endothelial cells)
Late effects on nl tissues of these higher radiation doses remain a concern
Radioprotectors are often scavengers of IR-induced free radicals. The most well studied is amifostine, which reduces xerostomia in head & neck cancer pts. However, its use has been limited due to concerns of diminished antitumor effects.
Radiosensitizers are actively being studied & may act by targeting the hypoxic cells or radioresistant clonogens w/in a tumor
Chemotherapy is frequently used sequentially or concurrently w/radiotherapy to maximize therapeutic benefit, although also a/w ↑ overall toxicity.
Drugs that show significant synergy w/RT: Dacarbazine, cisplatin, bleomycin, dactinomycin, Doxorubicin, mitomycin C, 5-FU, capecitabine, Gemcitabine, bevacizumab, cetuximab, PARP inhibitors
Mechanisms for synergy vary widely: Include cell cycle effects, hypoxic cell sensitization, & modulation of the DNA damage response
Due to cell killing of nl tissues (eg, dermatitis, esophagitis, & diarrhea), or by radiation-induced inflammatory cytokines (eg, nausea, vomiting, & fatigue)
Testes: 0.1-0.15 Gy leads to temporary sterility. Doses of 6-8 Gy can lead to permanent sterility. Such doses have min. effect on testosterone production.
Occur after a delay of mos to y, & can result from a combination of vascular damage and/or loss of parenchymal cells in the affected organ
Specific dose-volume relationships have been linked to the risk of late organ toxicity. Some of these data are summarized below, derived from the QUANTEC project (Int J Radiat Oncol Biol Phys 2010;76:S1-S160).
| ||||||||||||||||||||||||||||||
Dose, volume, underlying genetics, & age of the pt at the time of RT are critical determinants of the risk for secondary malignancy
The likelihood of secondary cancer is correlated w/dose, but there is no threshold dose below which there is no additional risk of secondary malignancy
Latent period for radiation-induced solid tumors is generally between 10 and 60 y, although exceptions are possible. Latent period for leukemias (less common after modern RT) is shorter—w/a peak between 5 and 7 y.
Important to distinguish relative risk from absolute risk when considering the likelihood of secondary malignancy
EBRT = therapeutic RT delivered by an external machine to treat malignancies & some benign conditions
Contrast w/brachytherapy: RT delivered by a source inside/near the tumor
| ||||||||||||||||||
Consultation visit: Radiation oncologist evaluates need for RT, schedules simulation
Pt immobilized using variety of devices
Isocenter & alignment tattoos placed on the pt
Images transferred to a treatment-planning computer
Set volumes: MD contours target volumes & nl structures on the planning CT scan; if simple 2D treatment, MD also places desired RT beams on the CT
Write prescription: MD prescribes a specific dose & fractionation of RT
Treatment planning:
If 2D, physics staff verifies the dose & beams, & EBRT can begin quickly
If 3D or more complex treatment, physics staff generates custom plan unique for each pt; can take up to 1 wk of treatment planning & QA review
Treatment plan reviewed & approved by MD
Pt set-up on treatment machine: A “dry-run” session to double-check all treatment & machine parameters
Conventional: 2D RT w/2 beams; used for simple treatments & lower total doses of EBRT eg, whole-brain RT, palliation for bone mets
3D conformal RT (3DCRT): 3D treatment plan using more than 2 beams to conformally treat a target region eg, pelvis for rectal cancer
IMRT: Use of an inverse-planning algorithm to create a computer-generated 3D RT plan using multiple beam angles; optimizes treatment of target tissue while sparing critical nl tissues eg, head & neck cancers, prostate cancer. Requires contouring/delineation of both treatment volumes & nl tissues
Image-guided radiation therapy (IGRT): Daily or weekly 2D or 3D imaging to ensure treatment is precisely delivered; often used along w/IMRT, stereotactic radiosurgery (SRS), & stereotactic body RT (SBRT) to allow smaller margins on treatment volumes
4D CT: Used to account for pt internal motion from breathing, ensuring that tumor volume is not outside of the beams during treatment eg, lung cancer, upper abdominal cancers
Respiratory gating: Technique used to deliver RT only when a pt’s breathing falls w/in certain phases of respiratory cycle. Used for upper abdominal tumors.
Units of RT dose = Gy = 1 J/kg
Fractions = Number of sessions over which the total RT dose is delivered
Dose & fractionation are determined according to multiple factors: Intrinsic tumor radiosensitivity, proximity of tumor to critical nl tissues, data from randomized trials, use of sequential or concurrent chemotherapy, pt convenience
Typically, RT is delivered in daily fractions over a number of wks
Fractionation terminology:
| ||||||||||||||||||||||||||||||||||||
During RT, pts seen in clinic at least once a wk by attending MD. S/e are actively managed during these visits
Fatigue & mild skin erythema are most common acute s/e

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree