Some malignancies have been directly linked to alopecia. Mixed reports have appeared, indicating an association of prostate cancer with male pattern baldness. Some studies have shown that the risk of prostate cancer is as high as 50% in men who have male pattern baldness.7 Conversely, other studies have found no association between male pattern baldness and prostate cancer. In addition, follicular mycosis fungoides, a rare disease associated with a poor prognosis, should be differentiated from alopecia mucinosa when considered in the setting of alopecia. These illnesses should be carefully considered when a patient presents with hair loss.
The diagnosis of alopecia should begin with a thorough history and physical examination. Patients should be asked about the onset of alopecia. Drug-induced alopecia can be differentiated by considering the time elapsed since medication was initiated and alopecia started. In addition, the pattern of the hair loss should be considered as a physical finding that can help in etiology of the hair loss. Clinical examination revealing bitemporal recessions can help to narrow the diagnosis between telogen effluvium and androgenetic alopecia.
Several tests can be conducted if a diagnosis of telogen effluvium is being considered. A blood workup should include, but not be limited to, blood count, thyroid function studies, rapid plasma reagin, Venereal Disease Research Laboratory, antinuclear antibody, and zinc or other nutritional deficiencies. In addition, clinical tests can determine whether the patient exhibits telogen effluvium such as hair-pull test and phototrichogram.
The most important test that helps to differentiate chronic telogen effluvium and androgenetic alopecia is the 4-mm punch biopsy. Biopsy is used to obtain a ratio of the amount of hair that is in anagen and telogen. This test has a diagnostic accuracy of 98%.5 In addition, a biopsy helps to differentiate between a follicular mycosis fungoides and metastatic tumor.
Several treatments in varying stages of research and with varying efficacies can be used in the treatment of chemotherapy-induced alopecia (Table 140.2). They include scalp cooling, minoxidil, immunomodulator ammonium trichloro-9-dioxoethlyene-O,O′-tellurate (AS101), scalp tourniquet, α-tocopherol, pulsed electrostatic fields (ETG), and various immunomodulating compounds. The therapies fall into three general modes: decreasing blood flow to the scalp, pharmacologically protecting the hair bulb, and inactivating the chemotherapeutic agent locally.
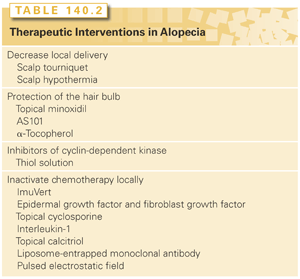
The amount of chemotherapy that reaches the scalp can be reduced in two ways: a scalp tourniquet or scalp hypothermia. Both methods induce vasoconstriction of superficial scalp vessels. For these methods to be effective, the chemotherapeutic agents must have short half-lives and rapid clearance of the drug and its metabolites.
Scalp tourniquets were first used in 1966. They are pneumatic devices placed around the hairline and inflated to a pressure greater than the systolic blood pressure while the patient is receiving the chemotherapy infusion. Several studies have been reported using scalp tourniquets that revealed their effectiveness in preventing hair loss; however, the studies are difficult to interpret due to inadequate sample size, inconsistent criteria of assessing alopecia, and differences in chemotherapy regimens. Side effects of the scalp tourniquet include nerve compression and headaches.4
Scalp hypothermia (i.e., scalp cooling), first used in 1978, decreases blood flow to the scalp and may slow uptake of the chemotherapeutic agent by the hair follicles. Several devices, such as bags of ice, molded gel packs, and thermocirculator devices, can be used to achieve scalp hypothermia; however, it is believed that the scalp temperature must be decreased to at least 24°C to effectively prevent alopecia. A common side effect is patient discomfort from heavy caps. Several concerns have been raised about the use of scalp hypothermia in those with liver dysfunction or in hematologic malignancies including leukemia and lymphoma, as well as a concern about the possibility of developing scalp metastases. More recent studies have found that hypothermia was effective, and several studies found no increased incidence of scalp metastases.8,9 A trial with 238 patients taking docetaxel found that scalp cooling reduced the risk of alopecia by 78%. The most common adverse event was sensation of cold.10 A study of 1,411 patients found that 50% of patients had good hair preservation. The study also showed positive benefits for almost all patients, except those using the taxotere, adriamycin, and cyclophosphamide regimen.11
There are currently no approved or effective pharmacologic treatments for chemotherapy-induced alopecia. Several different medications may reduce or prevent alopecia by pharmacologically protecting the hair bulb from the damaging effects of chemotherapy. These medications include AS101, topical minoxidil, α-tocopherol, thiol, and inhibitors of cyclin-dependent kinase (CDK).
AS101 is a synthetic compound that is structurally similar to cisplatin. Studies initially done with mice revealed that AS101 works as an immunomodulator by stimulating production of interleukin (IL)-1, IL-2, IL-6, colony-stimulating factor, and tumor necrosis factor, thereby potentially being used to minimize cytotoxicity. The mechanism of AS101 may be due to IL-1 because there is an inverse correlation between IL-1 and alopecia.12 An open-label prospective randomized trial with 44 patients who had unresectable or metastatic nonsmall-cell lung cancer and were receiving carboplatin and etoposide revealed a significant reduction in neutropenia and thrombocytopenia as well as chemotherapy-induced alopecia.13
Topical minoxidil has been used for the treatment of androgenetic alopecia and alopecia areata. Two randomized trials have been performed in patients with cancer. The first was in 48 patients with many different solid tumors who were receiving doxorubicin-containing regimens. Minoxidil 2% twice a day did not prevent alopecia as compared with placebo.14
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








