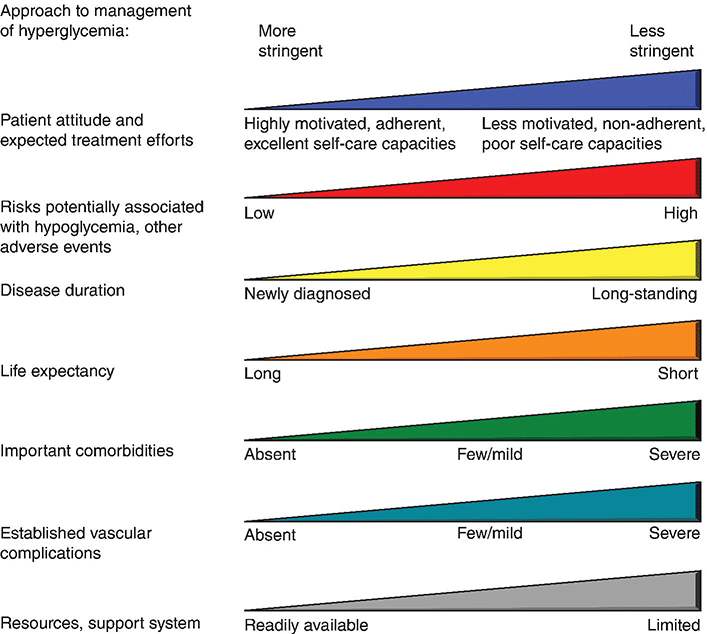
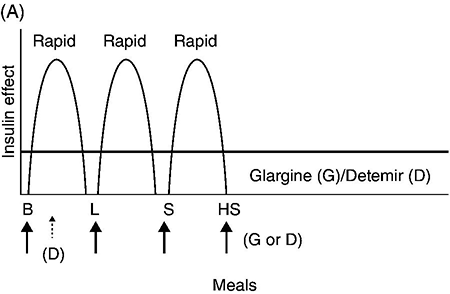
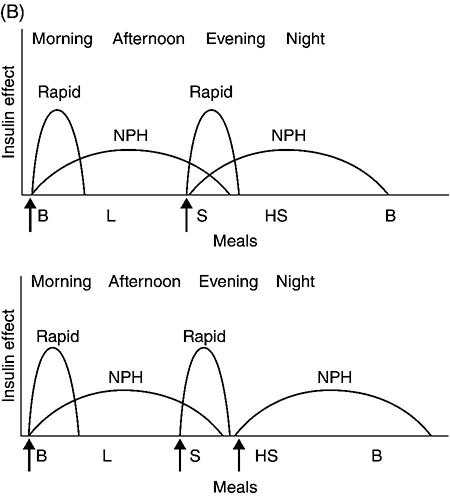
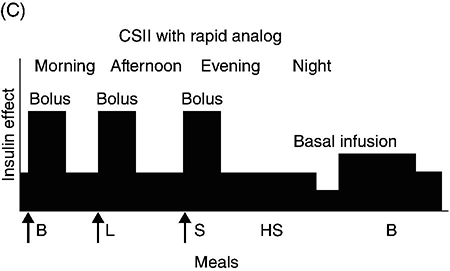
Chapter 4 Harpreet Kaur, MD and Ruth S. Weinstock, MD, PhD It has been well demonstrated that good blood glucose control can help prevent or slow the development of diabetes complications [1–5]. The approach to achieving glycemic goals and the pharmacological therapy used need to be individualized, and vary depending upon the type of diabetes, age, life expectancy, degree of hypoglycemic awareness, and presence of diabetes-related complications and other co-morbidities [6, 7]. Different treatment options are described, and their potential benefits and risks discussed in this chapter. Diabetes, more than any other chronic disease, requires daily self management. Lifestyle change is often recommended. A multidisciplinary team approach, providing comprehensive education and training as well as pharmacological therapy, can be particularly effective [8–13]. Team members may include, but are not limited to, physicians, dentists, physician extenders, dietitians, nurse educators, podiatrists, pharmacists, eye care providers, health coaches, social workers, and mental health professionals. Other specialists such as cardiologists, nephrologists, and neurologists may be required to assist with management of diabetes-related complications. Support from family, friends, and community can be especially helpful in attaining lifestyle change goals. To obtain the necessary instruction, people with diabetes and their family and friends should be referred to certified diabetes educators (CDEs) and/or comprehensive diabetes education programs that meet the National Standards for Diabetes Self-Management Education and Support [14]. Programs are certified by the American Diabetes Association (http://professional.diabetes.org/Recognition.aspx?typ=15&cid=84040) or the American Association of Diabetes Educators (http://www.diabeteseducator.org/ProfessionalResources/accred). Reimbursement can be available from Medicare and other insurers if the individual is referred to a certified program. Health care professionals interested in becoming CDEs are referred to the website of the National Certification Board for Diabetes Educators (http://www.ncbde.org/). Every person with diabetes should have an individualized glycemic goal [6, 7, 15, 16]. Goals are defined by results from the following methods for assessing glycemic control: home self-monitoring of blood glucose (SMBG), continuous glucose monitoring (CGM), and hemoglobin A1c (HbA1c) levels. Home blood glucose monitoring devices, which use a small drop of blood to measure blood glucose levels, are readily available. Frequency and timing of SMBG is individualized and vary with different insulin regimens, taking into consideration patient-specific factors such as history of hypoglycemia and hypoglycemic unawareness. SMBG should be performed by all individuals who use insulin therapy. The use of SMBG for people with type 2 diabetes who are not treated with insulin is controversial [17–21]. Potential benefits include the detection of severe hypoglycemia (relatively rare in non-insulin-treated patients) and a better understanding of glycemic excursions which alert patients to the need to modify their diet and/or medical regimen. Patients should have the skills and the ability to work collaboratively with their health care team to use the information from SMBG to improve their glycemic status [18]. The arguments against using SMBG in non-insulin-treated patients with type 2 diabetes primarily concern the expense, inconvenience, and paucity of evidence directly linking SMBG with improved glycemic control [17, 19, 20]. Continuous glucose monitoring systems use subcutaneous electrochemical sensors to measure interstitial glucose levels every few minutes, 24 hours/day. Glucose values are sent wirelessly to a receiver (a separate external device or an insulin pump). Individual values and trend arrows and graphs are displayed on the receiver, and alarms are used to warn users of serious hypoglycemia and hyperglycemia. By displaying glucose levels and trends every few minutes, patients are provided with important information that allows them to make better decisions regarding insulin dosing, carbohydrate intake, and physical activity. CGM systems need to be calibrated daily with SMBG and are not as accurate as SMBG, and values may lag behind SMBG when glucose concentrations change rapidly. Candidates for personal CGM systems are motivated individuals using intensive insulin regimens, primarily adults with type 1 diabetes who are willing and able to use the CGM system regularly as directed and make changes based on the CGM data [22–24]. CGM may particularly help people with a history of severe hypoglycemia, nocturnal hypoglycemia, or hypoglycemic unawareness [25]. Professional CGM systems are also available. These systems are purchased by the physician’s office, and are given to patients to wear for several days. Unlike the personal CGM systems, the patients cannot see their glucose levels in real time. When the devices are returned to the physician’s office, the CGM data are downloaded and reviewed. Changes in pharmacologic as well as non-pharmacologic therapies are often recommended based upon this information. The A1c test measures glycated hemoglobin, an indicator of the average blood glucose level over the past two to three months (Table 4.1). Higher A1c levels are associated with the development of more diabetes-related complications [1–5]. The standard of care is to obtain this test two to four times annually [6]. Blood samples can be sent to a certified clinical laboratory, or the test can be performed in the office using a small drop of blood in a point-of-care device. Table 4.1 Correlation of A1c with average glucose (AG). Establishing individualized goals requires the consideration of many factors, including the patient’s age, type of diabetes, duration of diabetes, presence of co-morbidities including microvascular and macrovascular diseases, degree of hypoglycemic awareness, life expectancy, financial constraints, and psychosocial issues (Figure 4.1) [6, 7, 15, 16]. The 2013 Standards of Medical Care in Diabetes, published by the American Diabetes Association recommend the following [6]: Figure 4.1 Approach to management of hyperglycemia. With permission from: Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012. 35:1366 People with type 1 diabetes have an absolute deficiency of insulin production and depend upon insulin therapy. This usually involves self-administration of at least 4 subcutaneous insulin injections daily (one to two injections of a basal insulin and mealtime injections of rapid-acting insulin; Figure 4.2A), or use of an insulin pump which is also referred to as continuous subcutaneous insulin infusion (CSII). If insulin therapy is interrupted or suspended in people with type 1 diabetes for a significant amount of time, they are at risk for developing life-threatening diabetic ketoacidosis. Figure 4.2 (A) Schematic representation of idealized insulin effect provided by three daily injections with rapid-acting insulin at meals and once-daily insulin glargine or detemir at bedtime. Detemir sometimes is given twice daily (dashed arrow). B = breakfast, L = lunch, S = supper, HS = bedtime, arrow = time of insulin injection before meals. (B) Schematic representation of idealized insulin effect provided by “split-mixed” insulin regimen consisting of two daily injections of rapid- and intermediate-acting insulin given before breakfast and supper (upper panel) and three daily injections with rapid- and intermediate-acting insulin before breakfast, rapid-acting insulin at supper, and intermediate-acting insulin at bedtime (lower panel). B = breakfast, L = lunch, S = supper, HS = bedtime, arrow = time of insulin injection before meals. (C) Schematic representation of idealized insulin effect provided by continuous subcutaneous insulin infusion (insulin pump) with rapid analog. B = breakfast, L = lunch, S = supper, HS = bedtime, arrow = time of insulin injection before meals. With permission from the American Diabetes Association [100]. Medical nutrition therapy (MNT; individualized nutrition counseling) is an essential aspect of management because specific diet-related behaviors influence glycemic control [29]. The mealtime insulin dosing depends in part on the carbohydrate content of that meal. Patients are taught carbohydrate monitoring (use of carbohydrate-consistent diets, experience-based carbohydrate estimations, or counting grams of carbohydrate). Nutritional recommendations are also based upon personal, social, cultural, and psychological factors; weight management considerations; and nutritional needs. In general, low-saturated fat (less than 7% of calories) and limited trans fat in the diet are recommended [6] to help reduce cardiovascular risk. Reimbursement of MNT by Medicare and many insurers is possible when provided by a registered dietitian. Regular physical activity (at least 150 minutes/week of aerobic exercise and twice/week resistance training) is recommended for people with diabetes who feel well and are not ketotic [6]. People with good glycemic control usually experience a fall in glucose levels with exercise, but glucose concentrations can rise in patients with uncontrolled diabetes (glucose greater than 250 mg/dL). The glucose response to exercise depends on the type, intensity, and duration of exercise; the serum glucose and insulin concentrations at the time of exercise; and the time, type, dose, and site of the recent insulin injections. Adjustments of insulin dosing and administration of carbohydrate-containing snacks are generally needed, particularly with long-term exercise [30]. Healthy individuals need to be educated in ways of avoiding hypoglycemia (reducing insulin dosing or adding carbohydrate before, during, and/or after exercise) prior to starting a new exercise regimen. Patients with cardiac disease, uncontrolled hypertension, severe neuropathy, untreated proliferative retinopathy, and other serious co-morbid conditions or cardiovascular risk factors should not begin an exercise program before consulting with their physician. SMBG is essential in the management of type 1 diabetes to help prevent serious hypoglycemia and hyperglycemia. It is recommended that people with type 1 diabetes self-monitor before meals and snacks; prior to, during, and after exercise; at bedtime; before driving or performing other potentially dangerous tasks; when under stress; when hypoglycemia or hyperglycemia are suspected; and occasionally after meals. Patients are taught to modify the timing and/or dosage of insulin and the timing and/or content of meals and snacks based on these glucose results. CGM can also be helpful in selected individuals. Individuals with type 1 diabetes require basal (continuous) insulin to keep glucose levels normal when not eating, and bolus (rapidly acting) insulin with meals to prevent post-prandial hyperglycemia. Bolus insulin is also used to correct hyperglycemia. The basal and prandial insulin requirements typically comprise 40–50% and 50–60%, respectively, of the total daily dose. This is accomplished with subcutaneous insulin injection therapy (usually at least four injections daily) or insulin pump therapy. Insulin therapy is adjusted for multiple factors, the most important of which are carbohydrate intake, physical activity, stress, and use of concomitant medications known to increase glucose levels (e.g., glucocorticoids). Insulin injections are administered using either a needle and syringe or an insulin pen. Injection therapy with insulin syringes requires drawing the amount of insulin needed from the vial into the syringe accurately. Because insulin is needed for meals and most snacks, a major limitation in the use of insulin vials, needles, and syringes is the inconvenience of carrying these items throughout the day. Insulin pens are pre-filled with insulin and may be either disposable or reusable. They are portable, easy to use, provide accurate dosing, may be particularly helpful for people with difficulties with vision and/or dexterity, and are associated with higher patient satisfaction compared to use of vials and syringes [31–33]. Insulin administration using syringes, however, is less expensive than using insulin pens. Insulin preparations are classified by duration of action: rapid-, short-, intermediate–, and long-acting (Table 4.2). Regular and neutral protamine hagedorn (NPH) insulin are the least expensive. They have the same amino acid sequence as human insulin. All other insulin preparations are insulin analogs, in which the insulin molecule has been modified to change its onset and duration of action. The pre-mixed insulins (Table 4.2) may be useful in selected patients with type 2 diabetes. Table 4.2 Insulin preparations available for subcutaneous use. Only regular insulin should be given intravenously. This is usually needed in hospital settings for the treatment of diabetic ketoacidosis, or for use in insulin infusions in inpatients who require insulin therapy and are not eating. Insulin glargine is a long-acting insulin analog that has the amino acid sequence of human insulin except for a substitution of glycine for asparagine in position A21 and the addition of two arginine molecules at the C-terminus of the B-chain of the insulin molecule. Because of these modifications, the insulin (in the vial or pen) needs to be at a lower pH to remain in solution. After injection into the subcutaneous tissue (neutral pH), microprecipitates form and small amounts of insulin are slowly released. This results in a prolonged duration of action with no pronounced peak effect. Insulin glargine is usually self-administered once daily (Figure 4.2A). Insulin detemir is a long-acting insulin analog that has duration of action that is partially dose-related, lasting longer when higher amounts are used. This analog differs from human insulin by the lack of threonine at position B30 and the addition of a fatty acid side chain at B29. Release from the subcutaneous tissue is slowed by self-association and albumin binding. A randomized trial comparing insulin glargine with insulin detemir for basal coverage in type 1 diabetes concluded that each provided similar effects on glycemic control (A1c levels) and risk of hypoglycemia, and there was no difference in tolerability [34]. Patients with type 1 diabetes require one to two injections of detemir daily for basal coverage (Figure 4.2A), and may require a higher dose of detemir compared to glargine. NPH (neutral protamine Hagedorn) insulin is an intermediate-acting insulin. It is neutral regular crystalline zinc insulin complexed to protamine; Hagedorn’s laboratory was the first to use protamine to prolong insulin action. NPH insulin is less expensive than the basal insulin analogs. NPH insulin needs to be used at least twice daily to provide 24-hour basal coverage, and has a significant peak activity, usually 6–8 hours after injection (Table 4.2, Figure 4.2B). Care is needed to avoid hypoglycemia during times of peak activity, which for morning dosing would occur in the afternoon, and for evening dosing could happen in the middle of the night. Administering NPH insulin at bedtime instead of before dinner can reduce the likelihood of middle-of-the-night hypoglycemia. The basal insulin analogs have been shown to be more effective than NPH in lowering fasting glucose levels in type 1 diabetes [35, 36]. Other intermediate-acting insulin preparations, primarily used in insulin mixtures, are shown in Table 4.2. Regular insulin has the same amino acid sequence as human insulin. It is complexed with zinc and self-associates into hexamers after injection. The hexamers need to dissociate into dimers and monomers to be absorbed into the bloodstream, prolonging its time of onset and duration of action (Table 4.2). Regular insulin is the least expensive short-acting insulin, but has the disadvantage that, when injected subcutaneously, has a slower onset of action and longer duration of action when compared to the rapidly acting insulin analogs. For this reason it should be injected at least 30 minutes before the meal and can cause hypoglycemia several hours after eating. There are three rapidly acting insulin analogs: aspart (aspartic acid at B28), lispro (proline B28 and lysine B29 are reversed), and glulisine (lysine at B3, glutamine acid at B29). These modifications result in faster absorption from the subcutaneous tissue resulting in a shorter duration of action (Table 4.2). The rapidly acting insulin analogs are routinely used for mealtime coverage in insulin injection therapy (Figure 4.2A). They are usually self-administered at the start of the meal or within 5–15 minutes of the start of the meal, with peak activity usually within one to two hours. In specific circumstances, such as when carbohydrate intake is uncertain, rapidly acting insulin is given right after the meal to avoid giving too much insulin, which would result in hypoglycemia. Some studies have shown better prandial glycemic control and lower risk of hypoglycemia with the use of insulin analogs [36]. The rapidly-acting insulin analogs are preferred for use in insulin pumps [37, 38]. Their higher cost (compared to regular insulin) is a disadvantage of the insulin analogs. Insulin pumps are widely used as an alternative method of delivering insulin in type 1 diabetes. The pump is an external device that contains rapidly acting insulin. CSII devices accurately deliver insulin, especially small doses, because fractions of a unit can be administered. The insulin is delivered subcutaneously through a disposable small catheter (infusion set) that is typically changed every two to three days by the patient. In most commercially available pumps, the catheter is connected by tubing to the pump which controls the rate of insulin delivery. The pump is typically placed in a clothing pocket or pouch. Alternatively, there are “tubing-free” CSII systems in which the insulin is placed in a “pod.” The pod is taped to the skin and directly delivers insulin subcutaneously through a small integrated canula. In the latter case, the device controlling the insulin infusion delivery communicates wirelessly with the pod. Some models of insulin pumps can receive SMBG and CGM values wirelessly. Insulin pumps deliver insulin every few minutes to provide basal insulin coverage. The basal insulin infusion rate(s) can be programmed either to deliver insulin at a constant rate over the 24-hour period or, commonly, to vary infusions rates at different times over the 24-hour period for patients who have different basal insulin requirements during the day or night (Figure 4.2C). Patients can also temporarily alter their basal rates. For example, during times of physical activity, basal rates can be reduced to avoid hypoglycemia. CSII devices can receive input (glucose levels and anticipated carbohydrate intake), and contain calculators that help patients determine their bolus doses. To control post-prandial hyperglycemia, boluses of insulin are delivered in proportion to the anticipated carbohydrate intake, typically using insulin:carbohydrate ratios. A bolus can also be used to correct hyperglycemia. To help determine the “correction dose,” the calculator uses the following insulin pump settings: glucose target, insulin on board (duration of active insulin), and “sensitivity factors” or “correction factors.” The latter are the glucose-lowering effect of one unit of insulin for that patient (e.g., one unit may lower glucose levels by 40 mg/dl). Pump systems also warn patients of insulin “stacking” (i.e., taking correction doses of insulin too close to each other which can results in later hypoglycemia). CSII is considered more flexible and convenient than injection therapy because patients do not have to carry insulin vials and syringes or insulin pens. A meta-analysis concluded that CSII, especially CGM (sensor)-augmented pump therapy, is associated with better glycemic control in adults with type 1 diabetes compared to SMBG with injection therapy [39]. In adults, quality of life was better with CSII, and in children, treatment satisfaction was higher with CSII treatment [39]. Disadvantages of insulin pump therapy are the following: The costs of the pump and pump supplies are higher than those of supplies needed for injection therapy. There is a small risk of infection at the insulin infusion site, especially if the infusion sets are not changed every three days. In addition, because only rapid-acting insulin is used in CSII, pump failure as a result of mechanical malfunction or catheter occlusion can quickly result in severe hyperglycemia and ketoacidosis. Patients treated with CSII must be motivated and properly trained to use pump therapy, should monitor their glucose levels frequently, and must always be prepared for the possibility of failure of the infusion system.
Glycemic treatment of diabetes mellitus
Introduction
Team approach
Glycemic goals
Assessments of glycemic control
Mean plasma glucose
A1c (%)
mg/dL
mmol/L
6
126
7.0
7
154
8.6
8
183
10.2
9
212
11.8
10
240
13.4
11
269
14.9
12
298
16.5
These estimates are based on ADAG data of approximately 2,700 glucose measurements over three months per A1c measurement in 507 adults with type 1, type 2, and no diabetes. The correlation between A1c and average glucose was 0.92 [99]. A calculator for converting A1c results into eAG, in either mg/dL or mmol/L, is available at http://professional.diabetes.org/eAG.
With permission from The American Diabetes Association. Standards of Medical Care in Diabetes 2013. Diabetes Care. 2013; 36 (Suppl 1): S19
Setting glycemic goals
Glycemic management of type 1 diabetes mellitus
Lifestyle
Home glucose monitoring
Insulin therapy
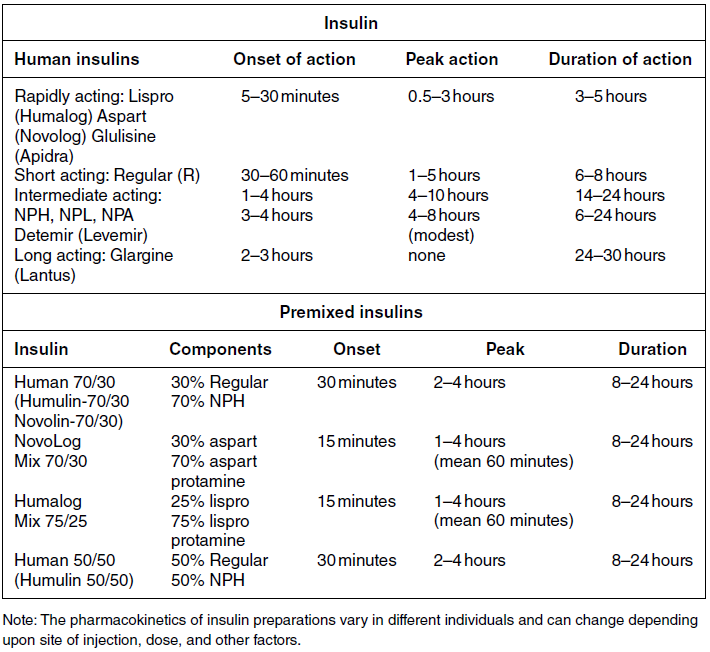
Basal insulin preparations
Bolus insulin preparations
Insulin pump therapy (continuous subcutaneous insulin infusion)
Pramlintide therapy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree