GLUCOCORTICOIDS
Part of “CHAPTER 78 – CORTICOSTEROID THERAPY“
STRUCTURE OF COMMONLY USED GLUCOCORTICOIDS
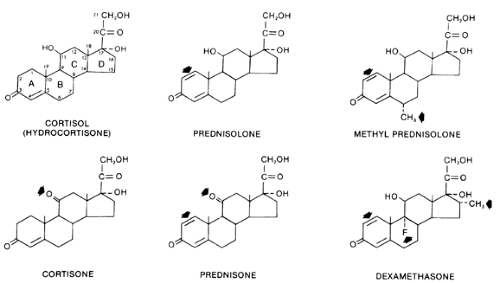
Figure 78-1 indicates the structures of several commonly used glucocorticoids.1,2 Cortisol (hydrocortisone) is the principal circulating glucocorticoid in humans.
Glucocorticoid activity requires a hydroxyl group at carbon 11 of the steroid molecule. Cortisone and prednisone are 11-keto compounds. Consequently, they lack glucocorticoid activity until they are converted in vivo to cortisol and prednisolone, the corresponding 11-hydroxyl compounds.3,4 This conversion occurs predominantly in the liver. Thus, topical application of cortisone is ineffective in the treatment of dermatologic diseases that respond to topical application of cortisol.4 Similarly, the antiinflammatory action of cortisone delivered by intraarticular injection is minimal compared with the effect of cortisol administered in the same manner.3 Cortisone and prednisone are used only for systemic therapy. All glucocorticoid preparations marketed for topical or local use are 11-hydroxyl compounds, which obviates the need for biotransformation.
PHARMACODYNAMICS
HALF-LIFE, POTENCY, AND DURATION OF ACTION
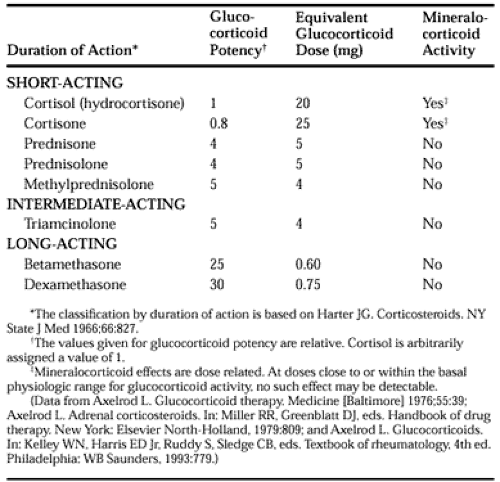
The important differences among the systemically used glucocorticoid compounds are duration of action, relative glucocorticoid potency, and relative mineralocorticoid potency (Table 78-1).1,2 The commonly used glucocorticoids are classified as short-acting, intermediate-acting, and long-acting on the basis of the duration of corticotropin (ACTH) suppression after a single dose, equivalent in antiinflammatory activity to 50 mg of prednisone (Table 78-1).5 The relative potencies of the glucocorticoids correlate with their affinities for the intracellular glucocorticoid receptor.6 The observed potency of a glucocorticoid, however, is determined not only by the intrinsic biologic potency, but also by the duration of action.6,7 Consequently, the relative potency of two glucocorticoids varies as a function of the time interval between the administration of the two steroids and the determination of the potency. In particular, failure to account for the duration of action may lead to a marked underestimation of the potency of dexamethasone.7
The correlation between the circulating half-life (T1/2) of a glucocorticoid and its potency is weak. The T1/2 of cortisol in the circulation is in the range of 80 to 115 minutes.1 The T1/2s of other commonly used agents are cortisone, 0.5 hours; prednisone, 3.4 to 3.8 hours; prednisolone, 2.1 to 3.5 hours; methyl-prednisolone, 1.3 to 3.1 hours; and dexamethasone 1.8 to 4.7 hours.1,7,8 Prednisolone and dexamethasone have comparable circulating T1/2s, but dexamethasone is clearly more potent. Similarly, the correlation between the circulating T1/2 of a glucocorticoid and its duration of action is poor. The many actions of glucocorticoids do not have an equal duration, and the duration of action may be a function of the dose.
The duration of ACTH suppression is not simply a function of the level of antiinflammatory activity, because variations in the duration of ACTH suppression are achieved by doses of glucocorticoids with comparable antiinflammatory activity. The duration of ACTH suppression produced by an individual glucocorticoid, however, probably is dose related.5
In short, the slight differences in the circulating T1/2s of the glucocorticoids contrast with their marked differences in potency and duration of ACTH suppression. Thus, the duration of action of a glucocorticoid is not determined by its presence in the circulation. This is consistent with the mechanism of action of steroid hormones. A steroid molecule binds to a specific intracellular receptor protein (see Chap. 4). This steroid-receptor complex modifies the process of transcription by which RNA is transcribed from the DNA template. This process alters the rate of synthesis of specific proteins. The steroid thereby modifies the phenotypic expression of the genetic information. Thus, the glucocorticoid continues to act inside the cell after it has disappeared from the circulation. Moreover, the events initiated by the glucocorticoid may continue to occur, or a product of these events (such as a specific protein) may be present after the disappearance of the glucocorticoid.
BIOAVAILABILITY, ABSORPTION, AND BIOTRANSFORMATION
Normally, a person’s plasma cortisol level is much lower after the oral administration of cortisone than after an equal dose of cortisol.9 Consequently, although oral cortisone may be adequate replacement therapy in chronic adrenal insufficiency, the oral form of this agent should not be used when larger, pharmacologic effects are sought. Comparable plasma prednisolone levels are achieved in normal persons after equivalent oral doses of prednisone and prednisolone.8,10 After the administration of either of these corticosteroids, however, there is wide variation in individual prednisolone concentrations, which may reflect variability in absorption.8
In contrast to the marked rises that follow the intramuscular injection of hydrocortisone, plasma cortisol levels rise little or not at all after an intramuscular injection of cortisone acetate. When it is given intramuscularly, cortisone acetate does not provide adequate plasma cortisol levels and offers no advantage over hydrocortisone delivered by the same route. The explanation for the failure of intramuscular cortisone acetate to provide adequate plasma cortisol levels is unknown. It may reflect poor absorption from the site of injection. Alternatively, intramuscular cortisone acetate, which reaches the liver through the systemic circulation, may be metabolically inactivated before it can be converted to cortisol in the liver, in contrast to oral cortisone acetate, which reaches the liver through the portal circulation.
PLASMA TRANSPORT PROTEINS
In normal humans, circadian fluctuations occur in the capacity of corticosteroid-binding globulin (transcortin) to bind cortisol and prednisolone. Patients who have been treated with prednisone for a prolonged period have no diurnal variation in the binding capacity of corticosteroid-binding globulin for cortisol or prednisolone, and both capacities are reduced in comparison with normal persons. Thus, long-term glucocorticoid therapy not only alters the endogenous secretion of steroids, but also affects the transport of some glucocorticoids in the circulation. This may explain why the disappearance of prednisolone is more rapid in those persons who have previously received glucocorticoids.
GLUCOCORTICOID THERAPY IN THE PRESENCE OF LIVER DISEASE
Plasma cortisol levels are normal in patients with hepatic disease. Although the clearance of cortisol is reduced in patients with cirrhosis, the hypothalamic-pituitary-adrenal (HPA) homeostatic mechanism remains intact. Consequently, the decreased rate of metabolism is accompanied by decreased synthesis of cortisol (see Chap. 205).
The conversion of prednisone to prednisolone is impaired in patients with active liver disease.11 This is largely offset by a decreased rate of elimination of prednisolone from the plasma in these patients.11 In patients with liver disease, the plasma availability of prednisolone is quite variable after oral doses of either prednisone or prednisolone.12 This is further complicated by the lower percentage of plasma prednisolone that is bound to protein in patients with active liver disease; the unbound fraction is inversely related to the serum albumin concentration. An increased frequency of prednisone side effects is observed at low serum albumin levels.12 Both these findings may reflect impaired hepatic function. Because the impairment of conversion of prednisone to prednisolone is quantitatively small in the presence of liver disease and is offset by a decreased rate of clearance of prednisolone, and because of the marked variability in plasma prednisolone levels after the administration of either corticosteroid, there is no clear mandate to use prednisolone rather than prednisone in patients with active liver disease or cirrhosis.8 If prednisone or prednisolone is used, however, a somewhat lower than usual dose should be given if the serum albumin level is low.8
GLUCOCORTICOID THERAPY AND THE NEPHROTIC SYNDROME
When hypoalbuminemia is caused by the nephrotic syndrome, the fraction of prednisolone that is protein bound is decreased. The unbound fraction is inversely related to the serum albumin concentration. The unbound prednisolone concentration remains normal, however.13,14 Because the pharmacologic effect is determined by the unbound concentration, altered prednisolone kinetics do not explain the increased frequency of predniso-lone-related side effects in these patients.
GLUCOCORTICOID THERAPY AND HYPERTHYROIDISM
The bioavailability of prednisolone after an oral dose of prednisone is reduced in patients with hyperthyroidism because of decreased absorption of prednisone and increased hepatic clearance of prednisolone.15
GLUCOCORTICOIDS DURING PREGNANCY
Glucocorticoid therapy is well tolerated in pregnancy.16 glucocorticoids cross the placenta, but there is no compelling evidence that this produces clinically significant HPA suppression or Cushing syndrome in neonates,16 although subnormal responsiveness to exogenous ACTH may occur. Similarly, there is no evidence that glucocorticoids increase the incidence of congenital defects in humans.16 Glucocorticoids do appear to decrease the birth weight of full-term infants; the long-term consequences of this are unknown. Because the concentrations of prednisone and prednisolone in breast milk are low, the administration of these drugs to the mother of a nursing infant is unlikely to produce deleterious effects in the infant.
GLUCOCORTICOID THERAPY AND AGE
The clearance of prednisolone and methylprednisolone decreases with age.17,18 Despite the higher prednisolone levels seen in elderly subjects compared with young subjects after comparable doses, endogenous plasma cortisol levels are suppressed to a lesser extent in the elderly.17 These findings may be associated with an increased incidence of side effects and suggest the need to use smaller doses in the elderly than in young patients.
DRUG INTERACTIONS
The concomitant use of medications can alter the effectiveness of glucocorticoids; the reverse also is true.19
EFFECTS OF OTHER MEDICATIONS ON GLUCOCORTICOIDS
The metabolism of glucocorticoids is accelerated by substances that induce hepatic microsomal enzyme activity, such as phenytoin,
barbiturates, and rifampin. The administration of these medications can increase the corticosteroid requirements of patients with adrenal insufficiency or lead to deterioration in the conditions of patients whose underlying disorders are well controlled by glucocorticoid therapy. These substances should be avoided in patients receiving corticosteroids. Diazepam does not alter the metabolism of glucocorticoids and is preferable to barbiturates in this setting. If drugs that induce hepatic microsomal enzyme activity must be used in patients taking corticosteroids, an increase in the required dose of corticosteroids should be anticipated.
barbiturates, and rifampin. The administration of these medications can increase the corticosteroid requirements of patients with adrenal insufficiency or lead to deterioration in the conditions of patients whose underlying disorders are well controlled by glucocorticoid therapy. These substances should be avoided in patients receiving corticosteroids. Diazepam does not alter the metabolism of glucocorticoids and is preferable to barbiturates in this setting. If drugs that induce hepatic microsomal enzyme activity must be used in patients taking corticosteroids, an increase in the required dose of corticosteroids should be anticipated.
Conversely, ketoconazole increases the bioavailability of large doses of prednisolone (0.8 mg/kg) because of inhibition of hepatic microsomal enzyme activity.20 Oral contraceptive use decreases the clearance of prednisone and increases its bioavailability.21
The bioavailability of prednisone is decreased by antacids in doses comparable to those used clinically.22 The bioavailability of prednisolone is not impaired by sucralfate, H2-receptor blockade, or cholestyramine.
EFFECTS OF GLUCOCORTICOIDS ON OTHER MEDICATIONS
The concurrent administration of a glucocorticoid and a salicylate may reduce the serum salicylate level. Conversely, reduction of the corticosteroid dose during the administration of a fixed dose of salicylate may lead to a higher and possibly toxic serum salicylate level. This interaction may reflect the induction of salicylate metabolism by glucocorticoids.23
Glucocorticoids may increase the required dose of insulin or oral hypoglycemic agents, antihypertensive drugs, or glaucoma medications. They also may alter the required dose of sedative-hypnotic or antidepressant therapy. Digitalis toxicity can result from hypokalemia caused by glucocorticoids, as from hypo-kalemia of any cause. Glucocorticoids can reverse the neuro-muscular blockade induced by pancuronium.
CONSIDERATIONS BEFORE INITIATING THE USE OF GLUCOCORTICOIDS AS PHARMACOLOGIC AGENTS
Cushing syndrome (see Chap. 75) is a life-threatening disorder. The 5-year mortality was higher than 50% at the beginning of the era of glucocorticoid and ACTH therapy.24 Infection and cardiovascular complications were frequent causes of death. High-dose exogenous glucocorticoid therapy is similarly hazardous.
Table 78-2 summarizes the important questions to consider before initiating glucocorticoid therapy.25 These questions enable the physician to assess the potential risks that must be weighed against the possible benefits of treatment. The more severe the underlying disorder, the more readily can systemic glucocorticoid therapy be justified. Thus, corticosteroids are commonly used in patients with severe forms of systemic lupus erythematosus, sarcoidosis, active vasculitis, asthma, chronic active hepatitis, transplantation rejection, pemphigus, or diseases of comparable severity. Generally, systemic corticosteroids should not be administered to patients with mild rheumatoid arthritis or mild bronchial asthma; such patients should receive more conservative therapy first. Although these patients may experience symptomatic relief from glucocorticoids, it may prove difficult to withdraw the drugs. Consequently, they may unnecessarily experience Cushing syndrome and HPA suppression.
DURATION OF THERAPY
The anticipated duration of glucocorticoid therapy is another critical issue. The use of glucocorticoids for 1 to 2 weeks for a condition such as poison ivy or allergic rhinitis is unlikely to be associated with serious side effects in the absence of a contraindication. An exception to this rule is a corticosteroid-induced psychosis. This complication may occur after only a few days of high-dose glucocorticoid therapy, even in patients with no previous history of psychiatric disease (see Chap. 201).26,27 Because the risk of so many complications is related to the dose and duration of therapy, the smallest possible dose should be prescribed for the shortest possible period. If hypoalbuminemia is present, the dose should be reduced. If long-term treatment is indicated, the use of an alternate-day schedule should be considered.
LOCAL USE
A local corticosteroid preparation should be used whenever possible because systemic effects are minimal when these substances are administered correctly. Examples include topical therapy in dermatologic disorders, corticosteroid aerosols in bronchial asthma and allergic rhinitis, and corticosteroid enemas in ulcerative proctitis. Systemic absorption of inhaled glucocorticoids leading to Cushing syndrome and HPA suppression is a rare occurrence when these agents are administered correctly at prescribed doses.28,29 The intraarticular injection of corticosteroids may be of value in carefully selected patients if strict aseptic techniques are used and if frequent injections are avoided.
SELECTING A SYSTEMIC PREPARATION
Agents with little or no mineralocorticoid activity should be used when a glucocorticoid is prescribed for pharmacologic purposes. If the dosage is to be tapered over a few days, a long-acting agent may be impractical. For alternate-day therapy, a short-acting agent that generally does not cause sodium retention (e.g., prednisone, prednisolone, or methylprednisolone) should be used. There is no indication for glucocorticoid conjugates designed to achieve a prolonged duration of action (several days or several weeks) after a single intramuscular injection. The bioavailability of such preparations cannot be regulated precisely, the duration of action cannot be estimated reliably, and it is not possible to taper the dosage rapidly in the event of an adverse reaction such as a corticosteroid-induced psychosis. The use of such preparations may cause HPA suppression more frequently than do comparable doses of the same glucocorticoid given orally. The use of supplemental medications to minimize the systemic corticosteroid dose and to reduce the side effects of systemic glucocorticoids should always be considered. In asthma, for example, treatment should include inhaled glucocorticoids and bronchodilators, such as β-adrenergic agonists and theophylline, and may include cromolyn.
EFFECTS OF EXOGENOUS GLUCOCORTICOIDS
ANTIINFLAMMATORY AND IMMUNOSUPPRESSIVE EFFECTS
Endogenous glucocorticoids protect the organism from damage caused by its own defense reactions and the products of these reactions
during stress.30,30a Consequently, the use of glucocorticoids as antiinflammatory and immunosuppressive agents represents an application of the physiologic effects of glucocorticoids to the treatment of disease.30 Glucocorticoids have many effects on inflammatory and immune responses, which are described in this section.
during stress.30,30a Consequently, the use of glucocorticoids as antiinflammatory and immunosuppressive agents represents an application of the physiologic effects of glucocorticoids to the treatment of disease.30 Glucocorticoids have many effects on inflammatory and immune responses, which are described in this section.
Glucocorticoids inhibit synthesis of almost all known cytokines and of several cell surface molecules required for immune function.31,32 and 33 When an immune stimulus such as tumor necrosis factor binds to its receptor, nuclear factor kappa B (NF-κB) moves to the nucleus, where it activates many immunoregulatory genes. This activation of NF-κB involves the degradation of its cytoplasmic inhibitor IκBβ and the translocation of NF-κB to the nucleus. Glucocorticoids are potent inhibitors of NF-κB activation. This inhibition is mediated by the induction of the IκBβ inhibitory protein, which traps activated NF-κB in inactive cytoplasmic complexes.31,32 and 33 This reduction in NF-κB activity appears to explain the ability of glucocorticoids to inhibit the production of cytokines and cell surface molecules and to suppress the immune response.
Influence on Blood Cells and on the Microvasculature.
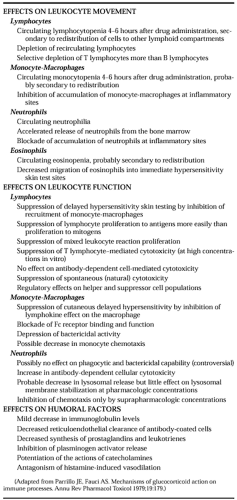
Glucocorticoid effects on inflammatory and immune phenomena include effects on leukocyte movement, leukocyte function, and humoral factors (Table 78-3). In general, glucocorticoids have a greater effect on leukocyte traffic than on function, and more effect on cellular than on humoral processes.34,35 glucocorticoids alter the traffic of all the major leukocyte populations in the circulation (see Chap. 212).
Probably the most important antiinflammatory effect of glucocorticoids is the ability to inhibit the recruitment of neutrophils and monocyte-macrophages to an inflammatory site.35 Cortico-steroids modify the increased capillary and membrane permeability that occurs in an area of inflammation. By decreasing the dilation of the microvasculature and the increased capillary permeability that occur during an inflammatory response, the exudation of fluid and the formation of edema may be reduced, and the migration of leukocytes may be impaired.2,35,36 The decrease in the accumulation of inflammatory cells is also related to decreased adherence of inflammatory cells to the vascular endothelium. It is not possible to determine the relative contributions of the direct vascular effect, the effect on inflammatory cell adherence to the vascular wall, and the effect on chemotaxis to the reduction in inflammation caused by glucocorticoids.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree