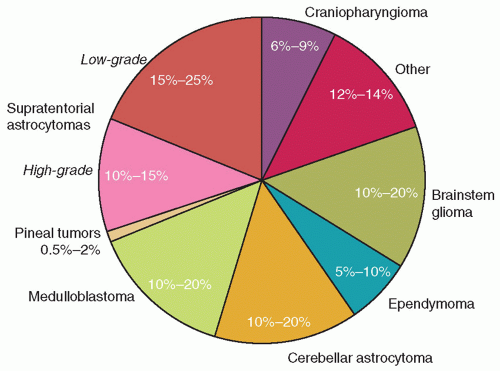
For most CNS tumors, surgical intervention forms the initial step in the treatment plan by providing tissue to establish the histologic diagnosis and, when possible, reducing the tumor burden. Notable exceptions to this rule are certain unresectable tumor types, such as diffuse infiltrative brainstem gliomas and globular chiasmatic gliomas in children with NF1. Each of these tumors has an almost diagnostic MRI appearance in association with consistent histologic features. As surgery has been shown not to improve prognosis in patients with these tumors and the histologic diagnosis rarely is in question, operative intervention is not required, although with the increasing adoption of molecularly targeted therapeutic strategies, stereotactic needle biopsy may in the future play a role in guiding protocol-directed therapy, particularly in brainstem gliomas, based on recent evidence that it can be accomplished with low morbidity.
70 Similarly, for certain deep-seated parenchymal tumors, the risks imposed by a conventional surgical approach to them may be high, and for establishing a histologic diagnosis, stereotactic biopsy techniques provide a reasonable alternative to open tumor debulking. CT- or MRI-guided stereotactic procedures are highly accurate in targeting such deep-seated lesions and are associated with a morbidity of less than 5% and a mortality of less than 1% of patients.
71,72
Preoperative and Perioperative Considerations
In occasional affected children who present with obtundation from a large mass lesion, resection is performed urgently. In children who are awake and alert but nonetheless harbor a large lesion that is producing substantial mass effect, the tumor resection is performed on the next operating day. Smaller lesions without significant mass effect can be managed more selectively.
Because peritumoral edema commonly contributes to the neurologic impairment produced by the tumor, moderate doses of corticosteroids generally are administered preoperatively. For example, 0.1- to 0.5-mg/kg dexamethasone may be given every 6 hours. This dosing often will lead to a dramatic improvement in the patient’s symptoms and signs, avoiding the need for emergency surgery in the vast majority of cases. Corticosteroids are typically continued intraoperatively and during the early postoperative period. If a significant reduction in tumor volume has been achieved at the time of operation, corticosteroid therapy is tapered and then discontinued within several days of surgery.
Another factor that commonly contributes to increased ICP in children with brain tumors is the presence of obstructive hydrocephalus, observed most commonly in tumors arising near the aqueduct, such as with pineal region tumors, or the fourth ventricle, as seen with cerebellar vermian lesions. Although resection of the tumor often opens the CSF pathways and leads to resolution of the hydrocephalus, the resection frequently is rendered safer if the elevated pressure is relieved as an initial step. The use of preoperative shunting carries a small risk of upward herniation through the tentorial hiatus. In some institutions, preoperative relief of hydrocephalus is accomplished by endoscopic third ventriculostomy; however, a more common approach is to place an external ventricular drain immediately before the craniotomy for tumor resection, which has the advantage of allowing drainage of bloody spinal fluid and debris in the early postoperative period. If the operative procedure opens the CSF pathways and the patient’s absorptive pathways remain patent, the external ventricular drain often can be removed within several days of surgery.
73 If the hydrocephalus persists, a third ventriculostomy can be performed or, if the hydrocephalus appears to be of a communicating type, a ventriculoperitoneal shunt can be inserted. Although in the past, a great concern was that shunting would provide a route for systemic dissemination of tumor, an increased risk of tumor spread via the shunt has not been demonstrated.
73The endocrinopathies commonly manifested by hypothalamic tumors can be exacerbated by tumor resection. Patients undergoing removal of tumors in this location typically require stress doses of hydroxycorticosteroids before, during, and after surgical intervention. Although thyroid hormone replacement is occasionally instituted preoperatively, it is most commonly initiated postoperatively. Patients in whom the posterior pituitary stalk is sectioned or injured during surgery often manifest a triphasic response of impaired fluid regulation, characterized by an initial period of transient diabetes insipidus lasting 1 to 2 days, a subsequent period of inappropriate antidiuretic hormone release lasting several days, and a final phase of persistent diabetes insipidus. In view of the rapid changes in vasopressin levels during the first several days postoperatively, careful attention to fluid replacement and cautious administration of synthetic vasopressin, where indicated, are essential to avoid potentially deleterious swings in electrolyte levels and fluid balance.
Children with cerebral cortical tumors and those in whom cortical retraction is required in the approach to a deep-seated lesion may be at risk for seizures during the perioperative period. Preoperatively, such patients often are started on an anticonvulsant medication (e.g., phenytoin) that is continued during the postoperative period, even if they have not experienced previous seizures. Patients generally are maintained on anticonvulsants for at least 1 week postoperatively; the decision to continue such therapy in patients without documented seizures is of uncertain benefit. In patients who experience a preoperative seizure disorder from the tumor and have been rendered seizure-free by tumor resection, anticonvulsants often can be stopped within several months after surgery.
Intraoperative Considerations and Surgical Technique
Multiple studies have demonstrated that the extent of surgical resection has a major impact on the likelihood of long-term survival
for children with many types of pediatric brain tumors, particularly ependymomas,
74 high-grade gliomas,
75,76 medulloblastomas (see
Chapter 26B), low-grade gliomas,
77 and choroid plexus tumors.
78 Accordingly, with the exceptions noted earlier, in which surgical resection is not indicated or in which stereotactic biopsy may be a preferable initial step, extensive resection is the goal for many types of pediatric brain tumors.
A major limitation to the widespread incorporation of extensive surgical resections in the management of childhood brain tumors has been the fact that aggressive resections may increase the risk of immediate- and long-term morbidity, particularly for tumors in functionally critical locations. Although morbidity generally is less than 10% for polar supratentorial gliomas and less than 20% for cerebellar astrocytomas, more deep-seated lesions,
such as ependymomas and craniopharyngiomas, carry morbidity rates in excess of 40% with extensive resection.
79 Although some studies have observed that morbidity is lower if operations are performed by neurosurgeons who do such operations frequently,
80 other studies show that pediatric neurosurgeons are more likely to attempt extensive removals, on the basis of their recognition that this influences prognosis, and therefore may have overall rates of management of morbidity comparable with those of general neurosurgeons, albeit with a higher frequency of complete or nearly complete tumor resections.
81During the last 10 to 20 years, a number of intraoperative modalities have been developed or refined to allow tumor resection to be performed more safely and efficiently. Foremost among these are the progressive improvements in operative microscopy, which facilitates illumination and visualization of the interface between the neoplasm and the normal brain. Localization techniques, such as frame-based and frameless stereotactic guidance systems, allow preoperative targeting of the tumor so that the surgical approach can be tailored precisely to minimize manipulation of normal brain structures and to maximize the extent of resection of deep-seated subcortical lesions.
82Ultrasonographic guidance also is useful in this regard. Intraoperative MRI units have become available in an increasing number of centers and may help to refine further the accuracy of intraoperative decision making,
83 provided that issues of cost and the difficulties involved in operating in or adjacent to a high-field-strength magnet can be resolved effectively.
In children whose tumors are in and around functionally critical brain regions, intraoperative monitoring of visual, auditory, and somatosensory pathways and direct assessment of motor and speech pathways often are used in an attempt to improve the safety of the tumor resection. In addition, areas of essential cortex overlying a deep-seated tumor may be delineated using cortical stimulation techniques to plan an approach to the tumor that avoids traversing important structures. Functional MRI and diffusion tensor imaging also provide a noninvasive way of delineating localizing important cortical and subcortical areas
84 to identify a safe trajectory to an underlying lesion. Finally, in children with intractable seizures from cerebral neoplasms, intraoperative or extraoperative electrocorticography (ECOG) may be used to define areas of epileptogenic cortex in and around the tumor to increase the likelihood that seizure control will be obtained postoperatively.
85For supratentorial craniotomies, children generally are placed in the supine or lateral position or prone for occipital lesions. For infratentorial craniotomies, the prone or lateral position is used more often than the “sitting” position because of concern over potential venous air embolism. Intraoperatively, the head of an infant often is positioned on a soft headrest, rather than being held by pins that can perforate the skull or cause a depressed fracture. For cortical and many subcortical tumors, the surgical approach follows the most direct trajectory to the lesion. However, for deep-seated lesions that are subjacent to functionally critical regions of the brain, alternate approaches often are required. Details of the operative approaches for specific tumor types are provided in subsequent tumor-specific sections of this chapter and Chapter 26B.
The actual tumor resection often is aided by the use of ultrasonic aspiration, which provides a relatively atraumatic way to debulk many pediatric brain tumors. The surgical laser also may be used, depending on the consistency and location of the tumor. In general, tumors are resected “from the inside out.” With many extra-axial tumors and a small percentage of intraparenchymal lesions, a clearly defined peritumoral plane is encountered through which the tumor may be dissected carefully from the surrounding brain, cranial nerves, and vessels after the central portion of the mass has been debulked. However, for most intraparenchymal tumors, a well-defined tumor capsule is not present, and the resection must proceed via gradual internal debulking until the boundary between the tumor and the normal brain is reached.
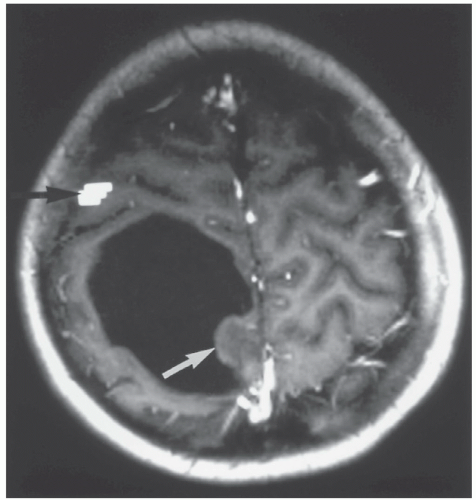
Because the extent of resection is so important in defining prognosis and choice of subsequent therapy for many tumor types, objective confirmation of the volume of residual tumor, if any, is essential before embarking on further therapy. Because a surgeon’s impression of the extent of tumor resection is subject to error, postoperative confirmation of the extent of resection generally is established by CT or, preferably, by MRI. This imaging typically is performed within the first 24 to 72 hours postoperatively to minimize the impact of postoperative inflammation on the delineation of areas of residual tumor.
A trend over the last decade in the surgical management of selected types of brain tumors has been the concept of second-look surgery. For large, relatively vascular tumors in which an initial complete resection cannot be obtained, the patient is treated with several courses of postoperative chemotherapy in the hope of making the tumor amenable to complete resection at a second procedure. This approach has been applied anecdotally in ependymomas, a tumor type in which the extent of residual disease before initiation of radiation therapy has a substantial impact on long-term outcome.
86 What remains to be determined is whether patients who undergo a second-stage complete resection have as good a prognosis as those who were amenable to complete resection initially; this issue is being examined systematically in studies of the Children’s Oncology Group (COG).
Surgical resection has been used increasingly as a component of the management of recurrent disease, particularly in children without evidence of tumor dissemination. For children with malignant lesions, this relieves mass effect in preparation for additional investigational chemotherapy on phase I or II clinical trials. Some recurrent tumors, such as JPAs and craniopharyngiomas, can be treated with reoperation alone, without the need for additional adjuvant therapy, if a gross-total resection (GTR) can be achieved. For children with recurrence of other, more malignant tumors that may also be subject to dissemination, data are lacking for a survival benefit from re-resection.
Postoperative Considerations
The postoperative recovery of patients who undergo a complete or partial resection of a posterior fossa tumor, particularly a tumor in or around the cerebellar vermis, may be complicated by posterior fossa syndrome. Posterior fossa syndrome, also known as “cerebellar mutism,” may range from a mild to severe disorder that is characterized by mutism, ataxia, hemiparesis, cognitive impairment, behavioral changes, cranial nerve palsies, bulbar palsy, and tremor.
87 The onset is typically within the first week after surgery and appears to occur more commonly with medulloblastoma than with other posterior fossa tumors.
88The etiology of the posterior fossa syndrome is unknown but appears to be associated with edema in the brachium pontis or with decreased blood flow during surgery. Previously reported as a rare complication of posterior fossa surgery, for unclear reasons, this syndrome is now observed in 10% to 25% of patients following tumor resection in the posterior fossa.
88 Although recovery may be complete, particularly for the mutism, there are patients in whom it is incomplete with long-term neurologic sequelae.
88,89