div class=”ChapterContextInformation”>
5. Parathyroid Glands
5.1 Calcium and Bone Metabolism
5.1.1 Roles of Calcium
Building block of bone, and helps maintain bone strength
Skeletal strength
Neuromuscular conduction
Stimulus secretion coupling (e.g., chromaffin cells)
The bones contain approximately 99.5% of the total calcium in the body.
5.1.2 Calcium in the Circulation
Ionized—biologically active (50%)
Complexed to citrate, phosphate, etc.—biologically active (5%)
Bound to protein, mainly albumin—inactive (45%)
Adjusted Ca = measured Ca + 0.02 × (40 − albumin) (where calcium is in mmol/L and albumin in g/L).
5.2 Calcium-Regulating Hormones
5.2.1 PTH and Parathyroid Glands
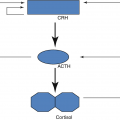
The central function of PTH is to regulate ionized [Ca 2+] levels by concerted effects on three principal target organs: bone, intestinal mucosa, and kidney.
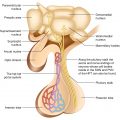
Parathyroid hormone (PTH) is an 84-amino-acid peptide secreted from four glands: the two superior glands are usually found near the posterior aspect of the thyroid capsule while the inferior glands are most often located near the inferior thyroid margin.
The normal parathyroid glands weigh an average of 40 mg each. Though the parathyroid glands are usually located on the back of the thyroid, their position is variable. Some people have one or more parathyroid glands elsewhere in the neck or in the chest—actually, they may be ectopic, located throughout the neck or upper mediastinum, along the thymus descending line. Moreover, 12–15% of normal persons have a fifth parathyroid gland. The explanation resides in their embryological development. The parathyroid glands arise from the third and fourth branchial pouches. The pair of glands which is ultimately inferior develops from the third pouch with the thymus, and one of them sometimes follows the thymus gland into the superior mediastinum whereas the pair of glands which is ultimately superior develops from the fourth pouch. These ectopic parathyroid glands may pose difficulties in localization when they become the site of a pathological process: PTH secreting adenoma formation.
5.2.1.1 PTH: Biologic Effects
On kidney—PTH has direct effects: ↑ Ca2+ reabsorption in the distal convoluted tubes, ↓ reabsorption of phosphate in the renal proximal tubule and of bicarbonate; additionally, ⊕ the synthesis of 1,25(OH)2D3 in the proximal tubule by inducing transcription of the 1α-hydroxylase gene.
On intestinal mucosa: the effect of PTH on intestinal calcium absorption is indirect, resulting from ↑ renal production of the intestinally active vitamin D metabolite, 1,25(OH)2D3.
On bone: PTH ↑ both bone formation and bone resorption; with regard to calcium homeostasis, the effect of PTH on bone resorption is dominant (Fig. 5.1).
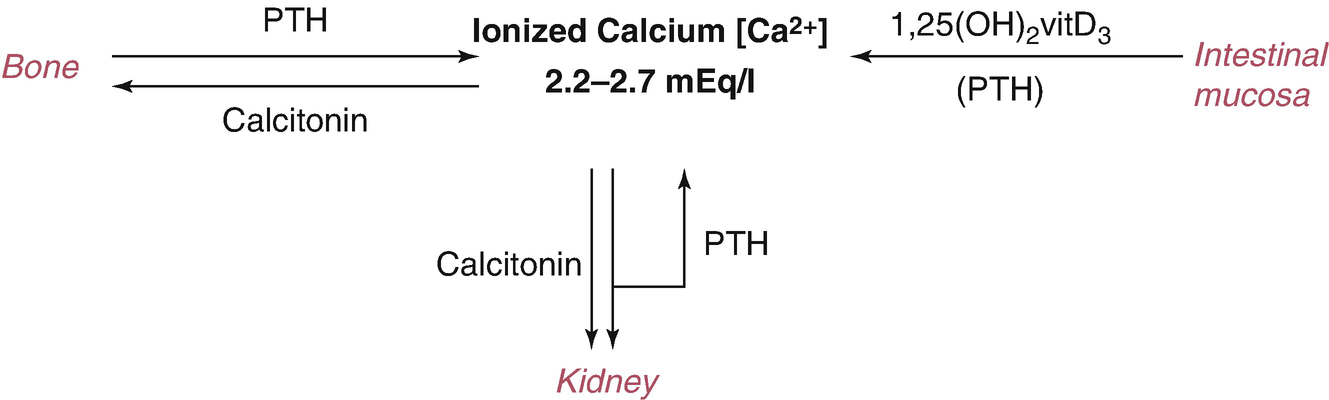
Hormones involved in calcium regulation
5.2.2 Calcitonin: Biologic Effects
On bone: CT inhibits osteoclastic-mediated bone resorption (its main function).
On kidney: CT inhibits the reabsorption of phosphate and ↑ the renal excretion of calcium.
Note: Calcitonin assays are available, but there is no role for calcitonin measurements in the routine investigation of calcium and bone metabolism. CT is of clinical interest for two reasons. First, CT is important as a tumor marker in medullary thyroid carcinoma (MCT). Second, CT has therapeutic uses as an inhibitor of osteoclastic bone resorption and it can be used in the treatment of Paget disease, hypercalcemia, and osteoporosis.
5.2.3 Vitamin D and Its Metabolites
The main function of vitamin D metabolites is the regulation of calcium and phosphate homeostasis, which occurs in conjunction with PTH. The gut, kidney, and bone are the principal target tissues for this regulation.
5.2.3.1 25OH Vitamin D (25OHD)
This is the main storage form of vitamin D, and the measurement of “total vitamin D” is the most clinically useful measure of vitamin D status. Internationally, there remains controversy around a “normal” or “optimal” concentration of vitamin D.
Levels over 50 nmol/L are generally accepted as satisfactory and values <25 nmol/L representing deficiency.
Low levels of 25OHD can result from a variety of causes (see Vitamin D deficiency). The major pathologic complication of vitamin D deficiency is rickets (in children) or osteomalacia (in adults), which results mainly from the deficiency of calcium and phosphate required for bone mineralization.
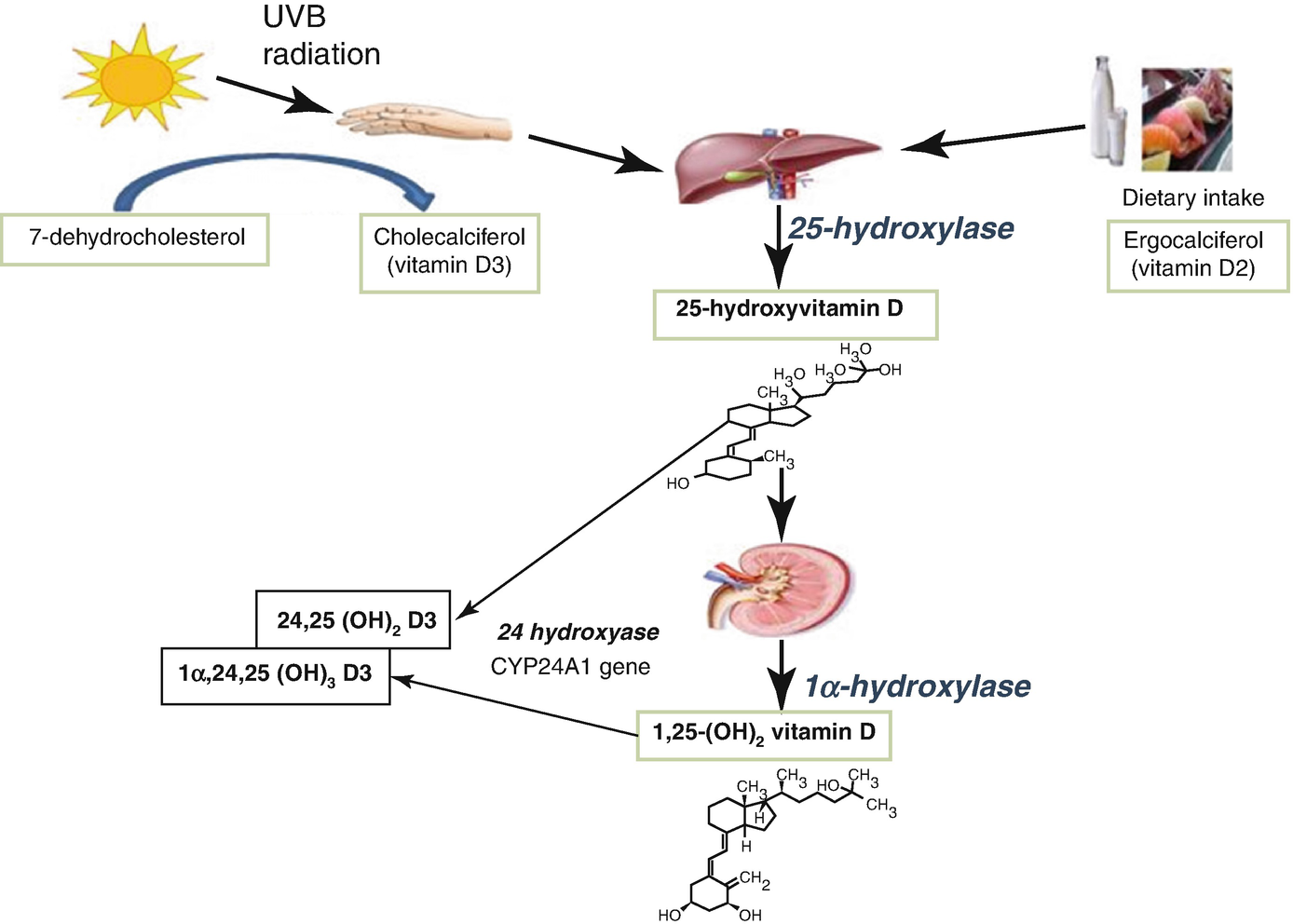
Vitamin D metabolism
5.2.3.2 1,25(OH)2 Vitamin D (1,25(OH)2D)
Although this is the active form of vitamin D, measurement of its concentration is rarely indicated. It is sometimes diagnostically useful in conditions of extrarenal synthesis of 1,25(OH)2D, such as in sarcoidosis.
Calcitriol (1,25(OH)2D3): Biologic Effects
On the intestinal mucosa: ⊕ calcium and phosphate reabsorption.
5.3 Regulation of Parathyroid Hormone Secretion
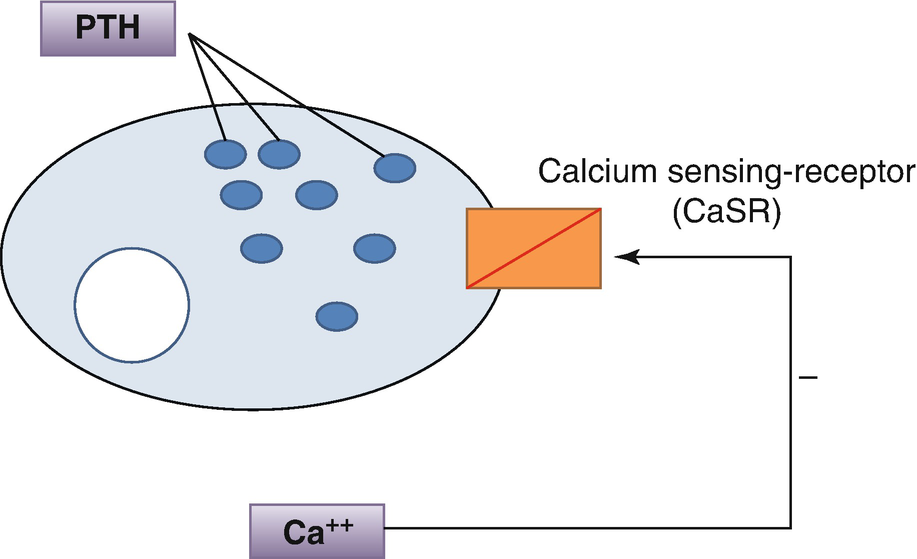
Regulation of parathyroid hormone secretion by Ca2+
5.4 Hypercalcemia
5.4.1 Epidemiology and Etiology
Hypercalcemia is found in 5% of hospital patients and in 0.5% of the general population.
Many different disease states can lead to hypercalcemia (see Table 5.1).
5.4.2 Investigation of Hypercalcemia
5.4.2.1 Confirm the Diagnosis
Serum calcium (adjusted for albumin)/Ca2+.
5.4.2.2 Determine the Cause
Causes of hypercalcemia
Common • Hyperparathyroidism: primary, tertiary • Malignancy: – Humoral hypercalcemia – Multiple myeloma – Bony metastases – Caused by PTH-related peptide (PTHrP) (solid tumors, adult T-cell leukemia syndrome) |
Uncommon • Vitamin D intoxication • Familial hypocalciuric hypercalcemia • Sarcoidosis and other granulomatous diseases • Thiazide diuretics • Lithium • Immobilization • Hyperthyroidism • Renal failure • Addison’s disease • Vitamin A intoxication |
Malignancy-associated hypercalcemia is the second most common form of hypercalcemia. Approximately 20% of patients with cancer develop hypercalcemia, which is due to humoral mechanism in 80% of patients and osseous metastases in the remaining 20%.
History and examination
Chest X-ray
FBC, ESR, and biochemical profile (renal and liver function)
Thyroid function tests (to exclude thyrotoxicosis)
25OHD (rarely 1,25(OH)2D), PTHrP
Plasma and urine protein electrophoresis (to exclude myeloma)
Serum cortisol (short Synacthen® test (to exclude Addison’s disease))
5.5 Primary Hyperparathyroidism
Hyperparathyroidism is by far the most common cause of hypercalcemia. Primary hyperparathyroidism results from excessive, autonomic secretion of PTH. Primary hyperparathyroidism is caused by a single parathyroid adenoma in about 80% of cases and by primary hyperplasia of the parathyroids in 10–15%. Parathyroid carcinoma is a rare cause of hyperparathyroidism, accounting for 1–2% of cases.
MEN type 1: parathyroid hyperplasia (primary hyperparathyroidism) + pancreatic tumors + pituitary tumors
MEN type 2a: medullary carcinoma of the thyroid + pheochromocytoma + parathyroid hyperplasia
5.5.1 Clinical Features
The typical clinical presentation of primary hyperparathyroidism has evolved considerably over the past few decades. As the disease continues to be detected primarily by multiphasic screening that includes determination of serum calcium levels, there has been a marked reduction in the frequency of the classic signs and symptoms of primary hyperparathyroidism, which are renal disease (stones, decreased renal function, and occasionally nephrocalcinosis) and the classic hyperparathyroid bone disease-osteitis fibrosa cystica.
In fact, about 85% of patients presenting today have neither bone nor renal manifestations of hyperparathyroidism and are regarded as asymptomatic or at most minimally symptomatic.
5.5.1.1 Bone Disease
Osteitis fibrosa cystica, the classic bone disease of hyperparathyroidism, causing bone pain and sometimes pathologic fractures; rare, usually with tertiary hyperparathyroidism; histologically, there is an increase in the number of bone-resorbing osteoclasts, marrow fibrosis, and cystic lesions that may contain fibrous tissue (brown tumors).
Osteoporosis (unlike other osteoporotic disorders, hyperparathyroidism often results in the preferential loss of cortical bone).
5.5.1.2 Hyperparathyroid Kidney Disease
Kidney stones: they are usually calcium oxalate stones (usually recurrent and difficult to manage medically).
Nephrocalcinosis: deposition of calcium in the renal medulla (clinically evident nephrocalcinosis rarely occurs, but a gradual loss of renal function is not uncommon).
Renal impairment: [eGFR] <60 mL/min.
Polydipsia and polyuria ← chronic hypercalcemia can also compromise the renal concentrating ability.
CNS effects: lethargy, depression, psychosis, ataxia, stupor, and coma.
Neuromuscular effects: weakness, proximal myopathy, and hypertonia.
Cardiovascular effects: hypertension, bradycardia (and eventually asystole), and a shortened QT interval.
Digestive effects: nausea, indigestion, vomiting, anorexia, constipation (sometimes even—ileus ←↓ bowel motility ← hypercalcemia), peptic ulcers, pancreatitis.
Eye findings such as band keratopathy.
Systemic metastatic calcification: skin → itch; joints → chondrocalcinosis.
5.5.2 Primary Hyperparathyroidism Workup
5.5.2.1 Diagnosis of Primary Hyperparathyroidism
↑ Ionized calcium or total calcium; serum phosphorus is low-normal or low (<3.5 mg/dL)
Urinary calcium ↑; urinary phosphorus ↑
↑ of ALP level—reflecting high bone turnover
Hormonal Determinations
↑ Intact PTH level (an elevated level of PTH is clearly inappropriate in a hypercalcemic patient and establishes the diagnosis of primary hyperparathyroidism); in a patient with ↑ PTH level, there is no need to screen for metastatic malignancy, sarcoidosis, etc.
↑ 1,25(OH)2D3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree