Abstract
Germ cell tumors (GCTs) are a very heterogeneous group of tumors that range from benign (teratoma) to highly malignant (choriocarcinoma). Many tumors contain mixed elements. This chapter describes the current diagnosis, staging, and treatment of GCTs. Coordination of chemotherapy and surgery is essential. With the advent of effective chemotherapy, significant improvement in survival for patients with GCTs has been achieved.
Keywords
Germ cell tumor, teratoma, immature teratoma, seminoma, germinoma, dysgerminoma, yolk sac tumor, embryonal carcinoma, choriocarcinoma, mixed germ cell tumor, sacrococcygeal teratoma
Germ cell tumors (GCTs) are neoplasms that develop from primordial germ cells of the human embryo, which are normally destined to produce sperm or ova. Primordial germ cells appear to originate in the yolk sac endoderm and migrate around the hindgut to the genital ridge on the posterior abdominal wall where they become part of the developing gonad. Figure 29.1 depicts the histogenesis of tumors of germ cell origin. Viable germ cells arrested along this path of migration may form neoplasia in midline sites, such as the pineal region (6%), mediastinum (7%), retroperitoneum (4%), sacrococcygeal region (42%) or in the ovary (24%), testis (9%), and other sites (8%).
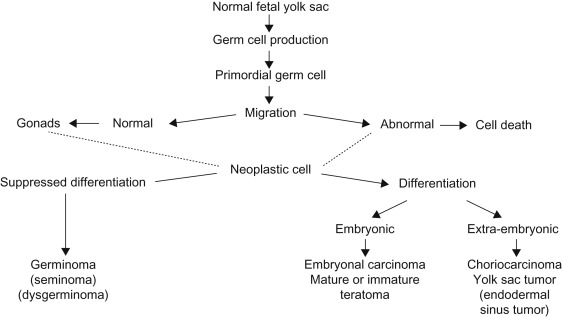
Incidence
Tumors of germ cell origin account for approximately 2–3% of childhood malignancies. The incidence of GCTs is 2.5 per million in white children and 3.0 per million in African-American children under 15 years of age with a male:female ratio of 1.0:1.1. GCTs are more common in the ovaries and testes than in extragonadal sites.
Pathology
The germ cells are the precursors of sperm and ova and retain the potential to produce all the somatic (embryonic) and supporting (extraembryonic) structures of a developing embryo. Yolk sac tumors ( YSTs ) ( endodermal sinus tumors ) are derived from a totipotential germ cell that differentiates to extraembryonic structures. Teratomas are embryonal neoplasms that contain tissues from all three germ layers (ectoderm, endoderm, and mesoderm). Teratomas are mature or immature and may occur with or without malignant germ cell elements (endodermal sinus tumors, choriocarcinomas, embryonal carcinomas, or germinomas) or rarely malignant somatic elements (such as primitive neuroectodermal tumors in children).
The malignant histologic variants of GCTs are:
- 1.
Germinoma.
- a.
Dysgerminoma (ovary).
- b.
Seminoma (testis).
- a.
- 2.
Immature teratoma.
- 3.
Embryonal carcinoma.
- 4.
YST (endodermal sinus tumor).
- 5.
Choriocarcinoma.
Table 29.1 describes the pathology of GCTs and associated biochemical markers.
| Histologic variant | Morphology | Common sites of origin | Markers a | ||
|---|---|---|---|---|---|
| AFP | β-hCG | PLAP | |||
| Germinoma |
|
| − | − | + |
| Mature teratoma |
|
| − | − | − |
| Immature teratoma |
|
| − | − | − |
| Embryonal carcinoma | Tumor cells polygonal with abundant pink vacuolated cytoplasm with ill-defined cellular membranes. Nucleus irregular and pleomorphic with prominent nucleoli. Cells arranged in solid sheets with scanty stroma or in tubules, acini, or papillary structures. Typical and atypical mitoses present | Testis (young adult) | − | ± | ± |
| Yolk sac tumor (endodermal sinus) |
|
| + | − | − |
Cytogenetic analysis has identified isochromosome 12p (i12p) as a specific abnormality in more than 80% of GCTs in postpubertal patients. Isochromosome 12p has also been identified in one-third of GCT in children under 5 years of age, suggesting that similar mechanisms for GCT generation may exist.
Clinical Features
The signs and symptoms of GCTs are dependent on the site of origin. Table 29.2 lists the clinical features of GCTs at different sites. In addition, certain histologic variants may have associated clinical findings, as listed in Table 29.3 .
| Tumor type | Median age year | Relative frequency (%) | Features |
|---|---|---|---|
| PEDIATRIC OVARIAN TUMORS | |||
| Dysgerminoma | 16 | 24 | Rapidly developing; 14–25% with other germ cell elements; very radiosensitive |
| Yolk sac tumor (endodermal sinus tumor) | 18 | 16 | ↑ AFP; 75% Stage I; all patients require chemotherapy because of high risk of relapse even in low-stage disease |
| Teratoma mature (solid, cystic) | 10–15 | 31 | Neuroglial implants may occur with cystic or solid teratomas, but do not affect prognosis; surgery is mainstay of treatment |
| Immature inversely | 11–14 | 10 | Grading system based on amount of neuroepithelium present; prognosis related to stage and grade; 30% with ↑ AFP |
| Embryonal carcinoma | 14 | 6 | 47% prepubertal; ↑β-hCG and precocious puberty common; chemotherapy indicated |
| Malignant mixed germ cell tumor | 16 | 11 | 40% premenarchal; 30% sexually precocious; AFP/β-hCG may be increased |
| Gonadoblastoma both | 8–10 | 1 | Associated with dysgenetic gonads and sexual maldevelopment; removal of gonads is treatment of choice |
| Other (polyembryoma, choriocarcinoma) | NA | <1 | Rare in children |
| PEDIATRIC TESTICULAR TUMORS | |||
| Yolk sac tumor (endodermal sinus tumor) | 2 | 26 | Most common of malignant germ cell tumors of the testes; ↑AFP; compared to adult cases, pediatric tumors are pure histologically; 85% Stage I; chemotherapy reserved for higher-stage or recurrent disease |
| Teratoma | 3 | 24 | Poorly differentiated histologic features do no impart a malignant course in children. Surgery alone is usually sufficient treatment |
| Embryonal carcinoma on stage | Late teens | 20 | Uncommon in young children; ↑AFP±β-hCG; managed as for adults, with retroperitoneal lymphadenectomy±chemotherapy±irradiation based |
| Teratocarcinoma | Late teens | 13 | 80% stage I with 75% survival after surgery alone; more advanced disease requires multimodality therapy |
| Gonadoblastoma | 5–10 | <1 | Associated with sexual maldevelopment syndromes; bilateral involvement in 30%; bilateral removal of gonads is treatment of choice |
| Other (polyembryoma choriocarcinoma) | NA | 16 | Rare in children |
| Histology | Clinical association |
|---|---|
| Teratoma | Musculoskeletal anomalies |
| Rectal stenosis | |
| Congenital heart disease | |
| Microcephaly | |
| Ovarian dysgerminoma (postpubertal) | Amenorrhea |
| Menorrhagia | |
| 46XY (male pseudohermaphrodite) | |
| Ovarian embryonal carcinoma | Precocious puberty |
| Amenorrhea | |
| Hirsutism |
Although GCTs are a diverse group histologically, all originate from primordial germ cells and have a common pattern of spread, irrespective of the primary site as follows:
- 1.
Lungs.
- 2.
Liver.
- 3.
Regional nodes.
- 4.
Central nervous system (CNS).
- 5.
Bone and bone marrow (less commonly).
Diagnostic Evaluation
The following evaluations should be carried out:
- 1.
History.
- 2.
Physical examination.
- 3.
Complete blood count.
- 4.
Liver function tests, electrolytes, blood urea nitrogen, creatinine.
- 5.
α-Fetoprotein (AFP).
- 6.
Human chorionic gonadotropin (β-hCG).
- 7.
Lactic dehydrogenase (LDH) isoenzyme 1. LDH may also correlate with disease activity such as:
- a.
Tumor bulk.
- b.
Residual tumor after surgery.
- c.
Response to chemotherapy and radiotherapy.
- d.
Tumor recurrence.
- a.
- 8.
Radiographic evaluation of primary site and regional disease.
- a.
Mediastinum: chest and upper abdominal computed tomography (CT).
- b.
Ovary: ultrasound, pelvic and abdominal CT or magnetic resonance imaging (MRI).
- c.
Sacrococcygeum: pelvic and abdominal CT or MRI.
- d.
Testis: ultrasound, pelvic and abdominal CT or MRI.
- a.
- 9.
Radiographic evaluation for distant metastases.
- a.
Chest radiographs (posteroanterior and lateral).
- b.
Chest CT.
- c.
Bone scan (may be reserved for patients with pure choriocarcinoma or known Stage IV disease).
- a.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







