Considerable progress has been made in recent years in understanding of the genetic basis for congenital neutropenia syndromes. With the advent of high-throughput genomic analyzing technologies, the underlying genetic causes of other congenital neutropenia syndromes are expected to be resolved in the near future. This knowledge will provide the foundation for genotype-phenotype correlations for infection susceptibility, response to therapy, and risk of malignant transformation, enabling optimal care for individual patients depending on their molecular pathophysiology. It is hoped that these investigations will enable the development of tailored molecular therapies to specifically correct the aberrant signaling cascades.
Key points
- •
Considerable progress has been made in recent years in understanding of the genetic basis for congenital neutropenia syndromes.
- •
With the advent of high-throughput genomic analyzing technologies, the underlying genetic causes of other congenital neutropenia syndromes are expected to be resolved in the near future.
- •
This knowledge will provide the foundation for genotype-phenotype correlations for infection susceptibility, response to therapy, and risk of malignant transformation, enabling optimal care for individual patients depending on their molecular pathophysiology.
- •
It is hoped that these investigations will enable the development of tailored molecular therapies to specifically correct the aberrant signaling cascades.
Introduction
Congenital neutropenia represents a heterogeneous group of inherited disorders with the common denominator of persistent and genetically determined paucity of neutrophil granulocytes in the peripheral blood. Neutropenia is termed mild when absolute neutrophil counts (ANCs) range from 1.0 to 1.5 × 10 9 /L, moderate with ANCs from 0.5 to 1.0 × 10 9 /L, and severe when ANCs are less than 0.5 × 10 9 /L.
Congenital neutropenia has to be distinguished from autoimmune neutropenias (usually self-limiting and not causing severe bacterial infections) and benign ethnic neutropenia (prevalent in the Middle East and Africa, not predisposing to infections). By contrast, congenital neutropenia leads to recurrent and severe bacterial (and sometimes fungal) infections. The classic phenotype of autosomal recessive severe congenital neutropenia (SCN) was described by the Swedish pediatrician Rolf Kostmann almost 60 years ago.
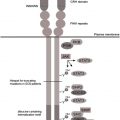
In the past, the genetic basis for several mendelian diseases with congenital neutropenia were elucidated and improved understanding of the molecular processes underlying this group of disorders ( Table 1 ). In the United States and Europe, most patients with SCN have either sporadic or autosomal dominance inheritance patterns and bear monoallelic mutations in the neutrophil elastase ( ELANE ) gene (see the article by Horwitz and colleagues elsewhere in this issue for further exploration of this topic). The molecular basis for autosomal recessive SCN is heterogeneous. The classic variant, originally described by Rolf Kostmann, is caused by mutations in the gene encoding for the mitochondrial protein HS1-associated protein (HAX1). In Europe, mutations in HAX1 seem to be more common than in the United States and account for up to 15% to 20% of cases of SCN (European Severe Chronic Neutropenia International Registry, unpublished data). Biallelic mutations in the glucose-6-phosphatase catalytic subunit 3 ( G6PC3 ) gene cause a complex disorder associating congenital neutropenia and various developmental aberrations such as congenital heart defects, urogenital malformations, and facial dysmorphy. Mutations in the GFI1 gene or activating mutations in the Wiskott-Aldrich syndrome ( WAS ) gene represent rare molecular causes of SCN. The relative frequency of these distinct gene defects among patients with SCN differs depending on ethnicity. In particular, in populations with a high degree of consanguinity, autosomal recessive variants of SCN are more prevalent than mutations in ELANE . Despite recent progress in the field, in around one-third of patients with SCN, no pathogenic mutations can be identified.
| Disease | Gene Mutated | Inheritance | Hematopoietic Manifestations | Extrahematopoietic Manifestations | Pathophysiologic Mechanism | References |
|---|---|---|---|---|---|---|
| (1) SCN | ||||||
| ELANE deficiency (SCN1) | ELANE | AD | CN or CyN | — | Activation of the UPR, excessive apoptosis of myeloid cells | |
| GFI1 deficiency (SCN2) | GFI1 | AD | CN, lymphopenia | — | Defective myeloid cell differentiation | |
| HAX1 deficiency (SCN3) | HAX1 | AR | CN | Epilepsy, neurologic impairment in some patients | Destabilization of mitochondrial membrane potential, excessive apoptosis of myeloid cells | |
| G6PC3 deficiency (SCN4) | G6PC3 | AR | CN, thrombocytopenia | Congenital heart defects, facial dysmorphy, increased visibility of superficial veins, urogenital malformations, failure to thrive, endocrine abnormalities, inner ear hearing loss, hyperelasticity of the skin | Impaired intracellular glucose homeostasis, activation of the UPR, excessive apoptosis of myeloid cells | |
| XLN | WAS | XL | CN, lymphopenia, myelodysplasia | — | Enhanced and discoordinated actin polymerization, defective cytokinesis | |
| (2) Congenital neutropenia: hypopigmentation disorders | ||||||
| Chédiak-Higashi syndrome | LYST / CHS1 | AR | CN, defective NK cell function, lysosomal inclusion bodies in leukocytes, macrophage activation syndrome | Oculocutaneous albinism, neurodegeneration | — | |
| Hermansky-Pudlak syndrome, type 2 | AP3B1 | AR | CN, impaired function of T and NK cells | Oculocutaneous albinism | Defective endosomal function | |
| Griscelli syndrome, type 2 | RAB27A | AR | CN, defective cytotoxicity, macrophage activation syndrome | Oculocutaneous albinism | — | |
| P14/ROBLD3/MAPBPIP deficiency | P14 / ROBLD3 / MAPBPIP | AR | CN, defective cytotoxicity, growth failure, lymphoid immunodeficiency | Oculocutaneous albinism | Aberrant distribution of late endosomes | |
| (3) Complex disorders comprising congenital neutropenia | ||||||
| AK2 deficiency | AK2 | AR | CN, severe lymphopenia (reticular dysgenesis) | Inner ear hearing loss | Aberrant mitochondrial metabolism caused by lack of adenylate kinase 2 | |
| Shwachman-Diamond syndrome | SBDS | AR | CN | Exocrine pancreatic insufficiency, skeletal dysplasia, hepatic and cardiac disease | Mitotic spindle destabilization, genomic instability, enhanced apoptosis | |
| WHIM syndrome | CXCR4 | AD | CN, myelokathexis, B cell deficiency | Warts, hypogammaglobulinemia, immunodeficiency | Constitutive activating mutations in CXCR4 associated with myelokathexis and lack of neutrophils in peripheral blood | |
| Poikiloderma with neutropenia | C16ORF57 | AR | CN, bone marrow abnormalities | Poikiloderma, increased photosensitivity | Disturbed biogenesis of U6 small nuclear RNA, impaired cell viability | |
| Cartilage-hair hypoplasia | RMRP | AR | CN, immunodeficiency | Hypoplastic hair, skeletal dysplasia | Defective assembly of ribosomes, aberrant cell cycle control and telomere function | |
| CD40L deficiency (HIGM1) | CD40L / CD154 | XL | Intermittent CN, combined immunodeficiency including T and B cell deficiency, defective B cell class switch (hyper-IgM syndrome type I) | — | Defective class switch caused by impaired CD40-CD40L interaction, defective T cell priming | |
| Barth syndrome | G4.5 / TAZ | XL | CN (in most patients) | Cardioskeletal myopathy, growth impairment, carnitine deficiency | Mitochondrial dysfunction, excessive apoptosis in myeloid cells | |
| Cohen syndrome | VPS13B / COH | AR | Intermittent CN | Psychomotor retardation, skeletal dysplasia, hypotonia | Suspected function of COH in vesicular transport processes | |
| Pearson syndrome | Deletion of mitochondrial DNA | mtDNA | CN, bone marrow failure | Exocrine pancreas insufficiency, endocrine abnormalities, neuromuscular degeneration | — | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree