Fig. 9.1
Incidence and mortality rates of female breast cancer by age, United States, 1975–2008 (Source: SEER 2012 [5])
Despite these recent promising trends, breast cancer represents a significant personal and societal burden that affects many women in the prime of their lives, accounting for a large portion of the oncology health-care budget. A long history of classical epidemiologic studies, now coupled with new information emerging from the field of molecular genetics, is beginning to elucidate the basic mechanisms of breast carcinogenesis and enable the development of novel prevention, detection, and treatment strategies.
Age
The risk of developing breast cancer increases throughout a woman’s lifetime, with it being relatively rare in younger women. Rates increase rapidly until age 50 and then continue to rise at a slower pace [6]. Despite the lower rates for young, premenopausal women that are less than 35 years old, they are more likely to be carriers of a breast cancer susceptibility gene. They also present with higher-grade tumors, a more advanced stage, and have a more biologically aggressive form of the disease, resulting in decreased disease-free and overall survival rates [7, 8]. The overall association of breast cancer incidence with increasing age is consistent with a stochastic model of breast cancer, wherein a progressive series of genetic changes within the cell lead to cancer initiation. It is becoming increasingly clear that these genetic changes are the result of a multitude of risk-related factors.
Race/Ethnicity
There are striking racial/ethnic differences in both the incidence and mortality rates for breast cancer. The incidence rates are highest for Caucasian women; lowest for Hispanic, Native American, and Asian American/Pacific Islanders; and intermediate for African-American women (see Fig. 9.2). However, despite having lower incidence rates than Caucasian women, African-American women have the highest death rates (see Fig. 9.3). Potential explanations for the discrepancies in rates among ethnic groups include environmental, societal, hormonal, and biological factors. Epidemiologic studies have consistently noted increased rates of breast cancer among Jewish women, an observation which is most likely explained by the recent identification of mutations in the BRCA1/2 genes which are more prevalent among individuals of Ashkenazi Jewish descent [9].
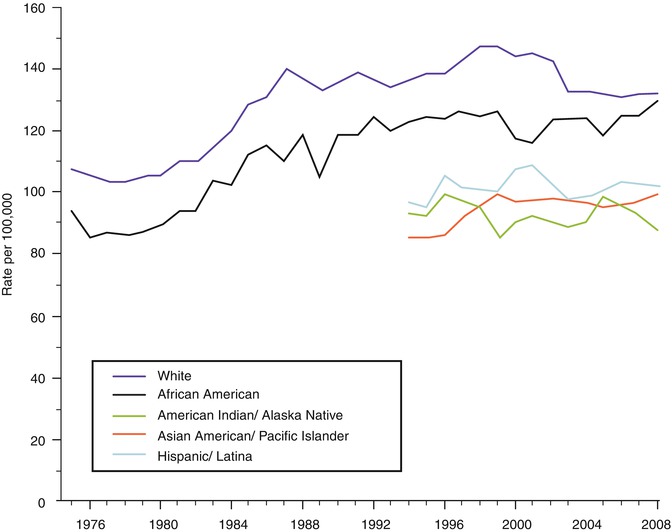
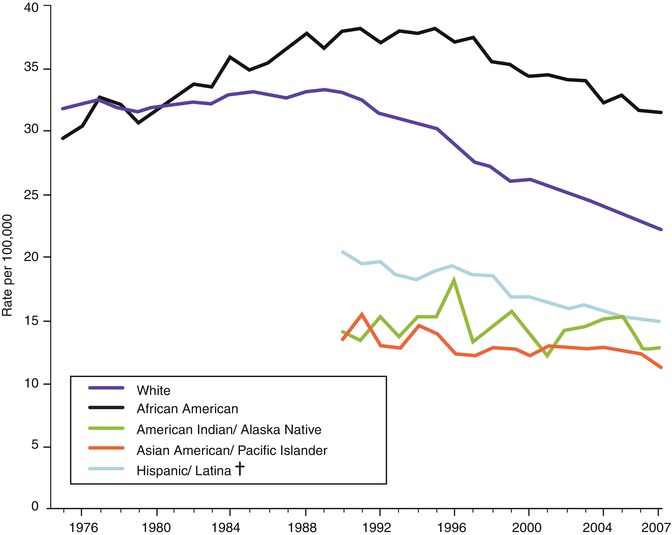

Fig. 9.2
Trends in female breast cancer incidence rates by race and ethnicity, 1975–2008 (Source: SEER 2012 [5])

Fig. 9.3
Trends in female breast cancer death rates by race and ethnicity, 1975–2007 (Source: SEER 2011 [4])
Country of Origin
The international patterns of breast cancer incidence reveal higher rates for Western, industrialized nations and lower rates for less industrialized and Asian countries (see Fig. 9.4). Furthermore, breast cancer incidence rates increase in migrants as they move from low-risk to high-risk countries [11]. These significant differences are thought to be attributable to variations in risk factors that are important in the etiology of the disease, such as reproductive practices, diet, differences in the availability of screening services [1], and perhaps genetic heterogeneity. While the long-term trend in increasing mortality rates for breast cancer has begun to be reversed in some countries, the trend is not universal, with mortality rates continuing to rise in India, South Korea, and Uganda [1]. Even within the US, there is significant geographic diversity in breast cancer rates, with mortality rates declining in the majority of states but stable in the South and the Western states [4]. Most of this variation is thought to be due to regional differences in breast cancer risk factors and variations in screening prevalence.
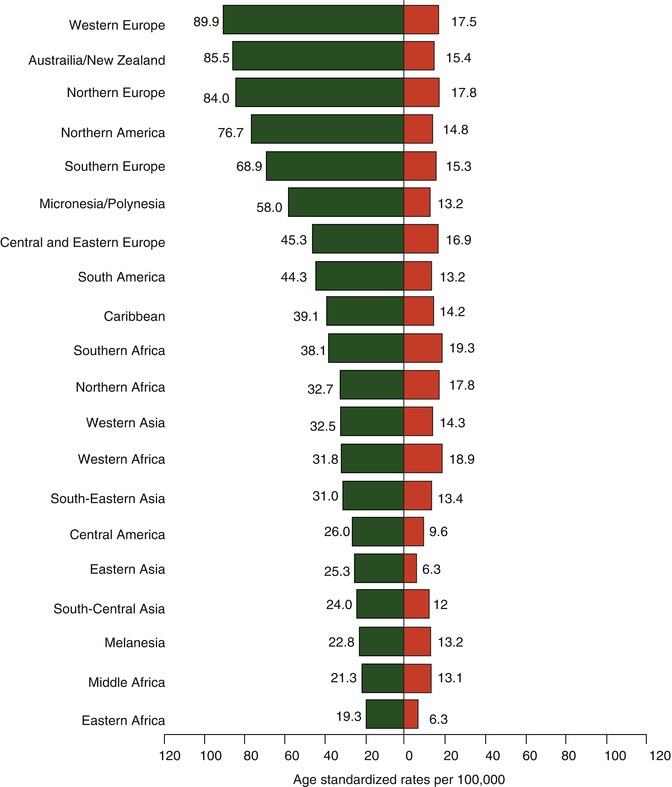

Fig. 9.4
Age-standardized breast cancer incidence and mortality rates by world area (Source: GLOBOCAN 2010 [10])
Benign Breast Disease
Fibrocystic breast disease has often been cited as a risk factor for subsequent breast cancer. However, careful evaluation of histologic patterns of benign breast disease has identified those groups in whom the risk is truly elevated. In the largest retrospective cohort study published, Dupont and Page [12] reevaluated over 10,000 consecutive biopsies performed over a two-decade time period, correlating histologic patterns with the subsequent incidence of breast cancer. Women undergoing breast biopsies, whose tissue showed no evidence of proliferation, had no increase in the relative risk of breast cancer. Risk increased from 1.4 to 4.4 with the degree of proliferation and atypia seen in the biopsy specimen.
There also appeared to be an interaction between the presence of proliferative breast disease and family and reproductive history, with relative risk increasing to as high as eightfold in the setting of a positive family history, nulliparity, or late age at first birth. This further suggests that the proliferative process itself is affected by other risk factors [12]. They also identified sclerosing adenosis and fibroadenomas as an independent risk factor for invasive disease, with relative risks in the 1.5–2.0 range [13, 14]. Confirmatory evidence of these findings comes from the prospective Nurses’ Health Study in which a prior diagnosis of atypical ductal hyperplasia or atypical lobular hyperplasia conferred an odds ratio of subsequent breast cancer of 2.4 and 5.3, respectively [15].
Lobular carcinoma in situ (LCIS) is the most common benign lesion to present as a multicentric and/or bilateral process and is often associated with sclerosing adenosis, stromal fibrosis, fat necrosis, duct ectasia, and fibroadenoma [16]. A diagnosis of LCIS increases the risk of subsequent breast cancer in either breast by a factor of 4–9 times the general population and is thought to be a marker of proliferation rather than a premalignant lesion itself [17]. The excess risk is highest for women who develop LCIS before the age of 40 years old [18].
Mammographic Density
The current data suggests an independent association between the degree of breast density seen on mammography and the risk for breast cancer. The increased relative risk for breast cancer ranges from 1.5 to sixfold among women in whom 75 % of the breast appears dense. Breast density is greatest in younger women; decreases with age, menopause, and pregnancies; and is thought to be a marker of proliferative activity [19, 20]. There also appears to be an additive effect of breast density and hormone exposure, with breast cancer risk higher among premenopausal women who have taken oral contraceptives (OC) and among postmenopausal women who took combined estrogen and progesterone hormone replacement therapy (HRT) [21, 22]. Breast density appears to be a heritable trait, with inherited factors accounting for 63 % of the variance [19].
Reproductive Risk Factors
One of the most consistent epidemiologic observations is the association of reproductive factors with the subsequent risk of breast cancer. One of the first observations noted an increased risk of developing breast cancer among nulliparous women. Not only was it noted that parous women had a decreased risk for breast cancer, but it was also observed that the degree of protection afforded by pregnancy depends on the age at the time of the first live birth (FLB), with the greatest protection seen among women whose FLB occurred before age 20 years [23]. In fact, when the FLB is delayed to age 35 or older, the risk for breast cancer equals, or exceeds, that of a nulliparous woman. The addition of subsequent pregnancies and/or lactation among young women adds additional protection. By causing breast epithelial cells to become fully differentiated and mature and less likely to undergo further mitoses, an early full-term pregnancy is thought to protect the cells from subsequent genotoxic events which may initiate the carcinogenic process.
An alternative hypothesis may be that pregnancy modifies age-related changes in plasma hormone levels [24, 25]. The total length of menstruation during a woman’s lifetime contributes to their overall risk. Both early age at menarche and late age at menopause are associated with an increased risk [26]. On the other hand, the risk for breast cancer is significantly decreased among women undergoing surgical oophorectomy, particularly if the surgery is performed before age 40 years [27]. The relevance of these findings appears to be related to the overall length of ovarian activity and the duration of exposure to ovarian hormones in the promotion of breast cancer.
An increased risk of breast cancer among postmenopausal women has been associated with the use of HRT. The Women’s Health Initiative examined postmenopausal women and randomized them to receive conjugated equine estrogen and medroxyprogesterone acetate versus placebo. The HRT group experienced significantly more cases of invasive breast cancer compared to the control group, HR 1.25 (CI 1.07–1.46), and were significantly more likely to present with lymph node-positive disease, HR 1.78 (CI 1.23–2.58). There were also significantly more deaths from breast cancer in the HRT group, HR 1.96 (CI 1.00–4.04) [28]. The resultant decline in use of HRT is thought to, in part, account for the decrease in breast cancer incidence seen in subsequent years.
Although the complex set of interactions which determine breast cancer risk are not fully known, there is a significant body of data linking factors associated with reproductive hormones to breast cancer incidence. This has led to the proposal that endogenous sex hormones are major determinants of breast cancer risk and that the role of other factors, including genetic polymorphisms, environmental exposures, and lifestyle events, may be in the modulation or metabolism of estrogen, progesterone, and/or androgenic hormones. Furthermore, this suggests that there is differential susceptibility of breast tissue to the adverse effects of hormone exposure, with the highest vulnerability found among less differentiated cells during periods of rapid growth. The lowest vulnerability is seen in cells which have undergone complete maturation during a full-term pregnancy. In this model, the initiation of cancer is seen as a multifactorial event, depending on the stage of development of the breast, the hormonal environment, the genetic susceptibility, and the presence or absence of modifying factors [29, 30].
Radiation Exposure
Epidemiologic studies have linked diagnostic and therapeutic ionizing radiation to subsequent breast cancer risk. Repeated diagnostic radiology procedures among adolescents and young women, for example, monitored for scoliosis or tuberculosis, have been associated with an increased risk for breast cancer that persists for decades after exposure [31]. Rates of breast cancer risk are also increased among women exposed to thymic radiation during infancy [32]. Perhaps the strongest radiation-associated risk for breast cancer is seen in women treated with chest radiation (mantle) for Hodgkin’s lymphoma. Among women treated before age 30 years, the risk for breast cancer is six times greater than that of the general population. Survivors of Hodgkin’s lymphoma are also more likely to experience contralateral breast cancers [33]. The degree of risk has been demonstrated to vary by age at treatment, time since diagnosis, and radiation dose [34].
Physical Activity
Both case-control and prospective studies have identified an inverse relationship between physical activity and breast cancer incidence. Reports of the risk reduction associated with moderate physical activity range from 15 to 40 %, and the effect is seen for both invasive and in situ breast cancer and among several ethnic groups [35, 36]. Several biological mechanisms have been proposed, including decreased body fat, decreased exposure to ovarian hormones, and decreased fasting insulin levels [37, 38].
Obesity
Obesity has been linked to an increased risk for breast cancer in both retrospective and prospective studies. The association of obesity with breast cancer risk is seen in Caucasian, African-American, and Asian women [39, 40]. The increased risk is best established among postmenopausal women, likely related to the interactions among cytokines, growth factors, inflammatory pathways, and steroid hormones [41].
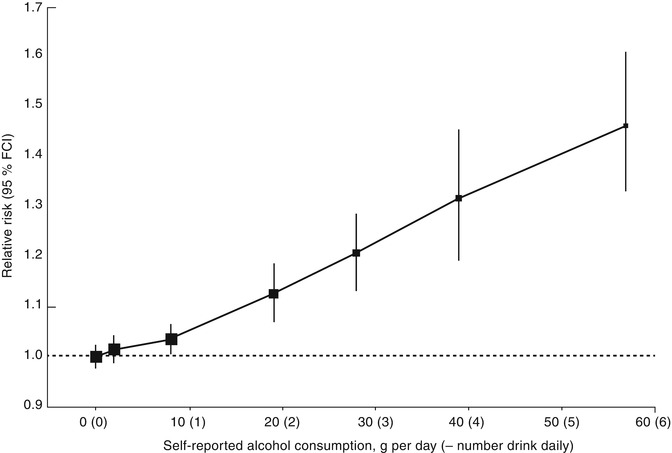
Alcohol
Several studies have revealed an association between alcohol consumption and breast cancer risk [42, 43]. The relative risk increases with increasing intake of alcohol [44] (see Fig. 9.5). The risk appears to be greater among women taking hormone therapy and is confined to breast cancers that express estrogen receptors, suggesting an interaction between alcohol and hormone pathways [45–47]. Alternative hypotheses are that alcohol is acting as a cumulative carcinogen and/or a tumor promoter [48].
Genetic Risk Factors
The basis of most, if not all, cancers involve one or more mutations in genes that are involved in the regulation of cell growth and/or DNA repair. Most cancer-related mutations are sporadic, i.e., they are acquired during the life of the individual and occur only in the tumor cells. However, the observation of familial clustering of certain cancers has led to the identification of a number of heritable forms of cancer in which the mutated gene is passed on from parent to child. We have now found several such genes that help to explain the clustering of breast cancer with other malignancies within family members. These hereditary breast cancer syndromes account for 5–10% of all breast cancer (see Table 9.1). They all share several features, including a Mendelian inheritance pattern, vertical transmission from both maternal and paternal lines, early age at onset, multiple primaries, and a high penetrance.
Table 9.1
Inherited breast cancer syndromes for which clinical testing is available
Syndrome | Involved gene(s) | Associated cancers |
|---|---|---|
Hereditary breast-ovarian cancer (HBOC) syndrome | BRCA1 at 17q21, BRCA2 at 13q12 | Breast, ovary, prostate, pancreas, melanoma |
Cowden syndrome | PTEN at 10q23 | Breast, uterus, thyroid, kidney, melanoma, glioblastoma |
Li-Fraumeni syndrome | TP53 at 17p13.1 | Sarcoma, breast cancer, leukemia, adrenocortical cancer, brain cancer |
Diffuse gastric cancer syndrome | CDH1 at 16q22.1 | Diffuse gastric cancer, lobular breast cancer |
Peutz-Jeghers syndrome | STK11 at 19p13.3 | Colon, breast, pancreas, uterus, lung, testis, and ovarian cancer |
Mutations in the BRCA1 and BRCA2 genes account for the majority of hereditary breast cancer. In addition to breast cancer, individuals with BRCA1/2 mutations are at an increased risk for cancer of the ovary and fallopian tubes, high-grade prostate cancer, male breast cancer, melanoma, and pancreatic cancer. Of the several hundred mutations described for the BRCA1/2 genes, most lead to a frameshift resulting in a missing or nonfunctional protein. These genes are known to be associated with a role as a “gatekeeper,” characterized by interactions with other genes in the regulation of the cell cycle and DNA repair [49].
The frequency of mutations in the BRCA1/2 genes in the general population has been estimated to range from 1 in 400 to 1 in 800. Carrier rates are not distributed evenly, however, and tend to concentrate in families with multiple cases of breast and/or ovarian cancer. BRCA1 and BRCA2 also share differential prevalence rates in certain ethnic groups. Most notably, three specific mutations, the 185delAG mutation and the 5382insC mutation on BRCA1 and the 6174delT mutation on BRCA2, have been found to be common among Ashkenazi Jews (AJ). The frequency of these three mutations approximates 1 in 40 in this population and accounts for up to 25 % of all AJ families with early-onset breast cancer and up to 90 % with both breast and ovarian cancer [9, 50]. Additional “founder effects” have been described in other populations.
The actual expression of disease in gene mutation carriers is known as the penetrance. Breast and ovarian cancer are the predominant cancers seen with BRCA1/2 mutations. The estimated risk for breast cancer by age 70 years is 47–66% for BRCA1 carriers and 40–57% for BRCA2 carriers. Ovarian cancer risk is estimated to be 35–46% for BRCA1 carriers and from 13 to 23 % among BRCA2 carriers [51, 52]. Among female BRCA1 and BRCA2 carriers who have already developed a primary breast cancer, estimates for a second contralateral breast cancer range from 39 to 56 %. Risks are higher among women whose first diagnosis was before age 40 and for women with BRCA1 mutations [53]. It is not known whether specific mutations within each gene confer differential rates of penetrance or what other genetic, environmental, or lifestyle factors may interact with the presence of a mutation to determine expressivity, thus making precise penetrance estimates in individuals somewhat difficult.
The phenotypic expression of BRCA1/2 breast cancer includes distinctive pathologic features. Breast cancers associated with the BRCA1 gene are typically estrogen receptor, progesterone receptor, and Her2/neu negative (triple negative), conferring an overall more aggressive disease course [54–56]. Sixty-nine percent of breast cancers arising in BRCA1 carriers are triple negative [54]. The prevalence of BRCA1 mutations among women with triple-negative disease has been reported to range from 11 to 35 % [57]. The phenotype for BRCA2-related tumors appears to be more heterogeneous and may include an excess of lobular histology [56].
Li-Fraumeni Syndrome
Breast cancer is also a component of the rare Li-Fraumeni syndrome (LFS) in which germline mutations of the TP53 gene on chromosome 17p have been documented. This syndrome is characterized by premenopausal breast cancer in combination with childhood sarcoma, brain tumors, leukemia and lymphoma, lung cancer, colon cancer, and adrenocortical carcinoma [58]. Individuals with LFS often develop multiple primary cancers [59]. A germline mutation in the TP53 gene has been identified in over 50 % of families exhibiting this syndrome, and inheritance is autosomal dominant with a penetrance of at least 50 % by age 50 [60].
Cowden Syndrome
One of the more than 50 cancer-related genodermatoses, Cowden syndrome is characterized by an excess of breast cancer, gastrointestinal malignancies, genitourinary malignancies, and thyroid disease (both benign and malignant). Skin manifestations include multiple trichilemmomas, oral fibromas and papillomas, and acral, palmar, and plantar keratoses. Germline mutations in PTEN, a protein tyrosine phosphatase with homology to tensin, located on chromosome 10q23, are responsible for this syndrome. Loss of heterozygosity observed in a high proportion of related cancers suggests that PTEN functions as a tumor suppressor gene. Its defined enzymatic function indicates a role in maintenance of the control of cell proliferation. Disruption of PTEN appears to occur late in tumorigenesis and may act as a regulatory molecule of cytoskeletal function. The lifetime risk of breast cancer in women with PTEN mutations is estimated at 25–50%, with a characteristic early age of onset [61].
Peutz-Jeghers Syndrome
Peutz-Jeghers syndrome (PJS) is characterized by gastrointestinal polyposis, particularly hamartomatous polyps, and melanocytic macules on the lips and perioral and buccal tissues [62, 63]. Although the most common cancers in PJS are gastrointestinal, the cumulative risk of breast cancer is 35–54% [63, 64]. Additional cancer risks include pancreatic and ovarian cancers as well as ovarian sex cord tumors with annular tubules (SCTATs) and mucinous tumors of the ovaries and fallopian tubes and Sertoli cell tumors of the testes [65, 66]. Germline mutations in the STK11 gene on chromosome 10p have been identified in the majority of PJS families [63, 67].
Hereditary Diffuse Gastric Syndrome
Hereditary diffuse gastric cancer (HDGC), which arises from mutations in the CDH1 gene, is associated with an increased risk of lobular breast cancer, with lifetime risks as high as 52 % and an average age of onset of 53 years old [68]. HDGC is unique in the predominant lobular pathology of the breast cancer occurrences as well as the diffuse nature of the gastric cancer cases. The risk for diffuse gastric cancer is >80 % in CDH1 gene carriers [69]. Loss of the E-cadherin protein causes the tumor cells to infiltrate the mucosa of the stomach, causing thickening and rigidity of the gastric wall, called linitis plastica, and malignant cells often have a signet ring appearance [70].
Lower-Penetrance Genes
In addition to the rare but highly penetrant genes described above, there is growing evidence that more common, but less penetrant, genes may account for a significant proportion of hereditary breast cancer. Both candidate gene and genome-wide searches have been used to identify low-penetrance polymorphisms that alone or in combination are associated with breast cancer. The identification and location of these breast cancer genes will ultimately permit further investigation of the precise role they play in cancer progression and will allow us to determine the percentage of total breast cancer caused by the inheritance of mutant genes. This, in turn, will ultimately enrich our understanding of all breast cancer, sporadic as well as hereditary, and will improve the identification of high-risk individuals.
Genetic Counseling for Breast/Ovarian Cancer
The technologic developments that have facilitated the location and isolation of cancer susceptibility genes have integrated the fields of oncology, cancer prevention, genetics, and counseling. This, in turn, has helped to create a new subspecialty of cancer risk counseling with an emphasis on the communication of risk information based on personal and family histories. The National Society of Genetic Counselors (NSGC) defines genetic counseling as the process of helping people understand and adapt to the medical, psychological, and familial implications of genetic contributions to disease [71]. Genetic counseling for cancer, also known as cancer risk assessment, is the important process of differentiating patients at low, moderate, or high risk for cancer and identifying appropriate patients for genetic testing of known hereditary cancer syndromes.
The specific goals of the counseling process are to (1) provide accurate information on the genetic, biologic, and environmental factors related to the individual’s risk of cancer; (2) provide a sufficient understanding of the genetic basis of breast and ovarian cancer to assist in decisions regarding genetic testing; (3) provide a realistic assessment of personal risk both for the genetic syndrome and for the disease(s) itself; (4) formulate appropriate options and recommendations for prevention and screening; and (5) offer psychosocial support, appropriate to a family’s culture, to facilitate adjustment to an altered-risk perception and promote adherence to the recommended actions. Using personal and family histories, risk models, and molecular and tumor testing, a counselor can help to identify patients at the highest risks for cancer [72]. As only 5–10% of cancer cases have a hereditary component, risk assessment can serve as a “gatekeeper” that identifies those patients most appropriate for high-cost genetic tests, ensuring tests and services are being utilized in a suitable manner. The session can also educate patients who are not appropriate for genetic testing but who still may have increased risks for cancer [73].
Target Population
With the growing awareness of the familial nature of breast cancer, increasing numbers of family members of women diagnosed with breast cancer are seeking information and advice about the disease, their potential risk, and available risk-reducing options. Women who seek counseling are often highly motivated by a personal experience with cancer in their family and by concern for the risks faced by themselves and their close relatives. As physicians become more aware of the importance of family history in determining a woman’s risk for breast cancer, they are increasingly referring their patients for cancer risk counseling. Even those individuals who are not candidates for genetic testing will benefit from having a realistic estimate of their cancer risk. In addition to providing risk information and risk counseling to the individual concerned about her risk for cancer, the process often has wide-reaching implications for the family and may ultimately extend to include additional family members.
The Counseling Team
Historically, the medical genetic counseling team has consisted of a medical geneticist, a genetic counselor, and, to a lesser extent, the referring primary care physician. As the field of genetic counseling has expanded to include adult diseases such as cancer, other disciplines, including oncology, gynecology, molecular genetics, social work, and psychology, have joined the team in order to provide the multidisciplinary approach needed.
There is also a growing interest in genetics on the part of nurses, many of whom are beginning to seek specialized training in the field. Genetic counselors and nurses trained in genetics are now increasingly delivering medical and genetic risk information about familial forms of cancer and counseling individuals and families about disease risk and management. Originally, cancer risk counseling programs were mainly situated in cancer centers and academic institutions, but these services are expanding to community hospitals, worksites, and health centers where they are often one component of a more broad-based health promotion program.
Risk Assessment
The genetic counseling process begins with the collection of several components of information. The first step in evaluating a woman’s risk for breast cancer is to evaluate her worries, questions, concerns, beliefs, and reasons for seeking counseling to guarantee that personal needs and priorities will be met in the counseling process. It is important at this early stage of counseling to establish a mutual trust and to negotiate a mutually agreeable agenda between the counselor and the individual seeking counseling. The counselor attempts to assess whether the individual’s expectations of cancer risk counseling are realistic. An exploration of the patient’s current knowledge of genetics, beliefs about the causes of cancer, and experiences with cancer can help to guide and personalize the session and ensure the clinician tailors their information for the patient. Throughout the course of the counseling process, the potential for psychological impact should be assessed and addressed. This includes evaluating for depression or anxiety, patient coping mechanisms and resilience, and available support systems (see Table 9.2). If necessary, referrals to mental health-care professionals should be provided [63].
Table 9.2
Components of information gathering
Establishment of needs, concerns, questions, priorities |
Assessment of psychosocial dimensions |
Personal medical history |
Detailed family history |
Personal risk factor profile |
Personal screening and health behavior history |
Family and Personal Health History
An expanded family pedigree that includes first-, second-, and third-degree relatives (parents, siblings, children, half-siblings, aunts, uncles, grandparents, cousins, etc.) using standard human pedigree nomenclature [74] begins the process of estimating cancer hereditary risks. Both maternal and paternal history should be evaluated for types of cancer, bilaterality, pathology, ages at diagnosis and/or death, and history of risk-reducing steps, such as chemoprevention or surgeries [75]. Ancestry/ethnicity and consanguinity information are also important when calculating likelihood of a genetic risk, because specific mutations (i.e., founder mutations) occur with increased frequency in certain populations.
It is important to include all relatives, both affected and unaffected, in the family history in order to judge the degree of penetrance of the disease. In the case of hereditary breast cancer, it is also important to document cases of ovarian and other cancers in the family. Limitations to family history, such as small family size, unknown history and adoption, early deaths precluding the possibility of developing adult-onset diseases, prophylactic surgeries which remove an organ from subsequent risk of cancer, or few family members of the gender most affected by the cancer syndrome risks, e.g., few women in a family at risk for hereditary breast ovarian cancer (HBOC) syndrome, can challenge the clinician’s ability to estimate hereditary risk [76]. Reported family histories may be inaccurate, and confirming documentation, such as pathology reports, should be obtained when possible [77, 78].
Family history data is then graphically represented on a pedigree, which follows standard nomenclature to illustrate family relationships and disease information in graphical format (Fig. 9.6). The hallmarks of features of a family history which suggest a hereditary pattern include multiple cancers in close relatives, multiple cancers in a single individual, multiple cancers across generations, early age of onset of cancer, bilateral cancer in paired organs (e.g., breast), the presence of precursor lesions known to be associated with the cancer phenotype, and ancestry [72] (see Table 9.3).
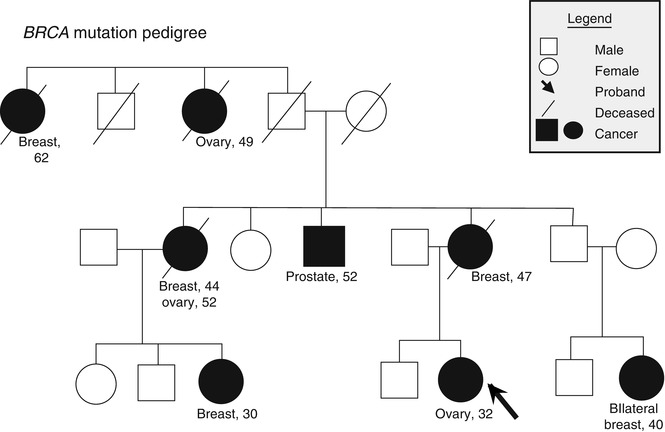

Fig. 9.6
BRCA mutation pedigree with standard nomenclature
Table 9.3
Features of hereditary cancers
Multiple cancers in close relatives |
Multiple cancers in a single individual |
Multiple cancers across generations |
Early age of onset of cancer |
Bilateral cancer in paired organs (e.g., breast) |
The presence of precursor lesions known to be associated with the cancer phenotype (e.g., multiple polyps in hereditary colon cancer) |
The patient’s personal medical history should also be ascertained at this time, including prior medical conditions, cancer diagnoses, and treatments. Additional information about carcinogen exposure, reproductive history, hormone use, lifestyle factors, and previous breast biopsy history will influence estimates of cancer risk. A record of past cancer screening practices establishes a history of health promotion behavior and will help guide the counselor in making reasonable and appropriate health recommendations. Physical findings associated with a hereditary breast cancer syndrome can also be evaluated during the risk assessment session [75]. In the absence of a clinician qualified to evaluate physical features, appropriate referrals to specialists should be made, e.g., dermatologic assessment of features of Cowden syndrome [61]. Beyond the data obtained during the creation of the family tree, this process allows the clinician to assess the client’s emotional state, become aware of family dynamics and level of support, and set the tone for the session [79]. This part of the session can also provide information about a patient’s perception of risk, which may be influenced by cultural and ethnic identity, family interaction, personal experience with the condition, and spirituality [79].
Risk Communication
The objective of risk communication is to provide patients with accurate information to help them understand and interpret their risk in order to make informed decisions about genetic testing and/or medical management [63]. Risk communication is particularly challenging in cancer genetic counseling due to the sheer number of different risks being shared with the patient (see Table 9.4).




Table 9.4
Risk communication
Cancer risks based on family history alone |
Cancer risks associated with carrying a gene mutation |
Risk the patient or family will test positive for a hereditary cancer syndrome |
Risk that other family members could carry the same genetic mutation if the patient tests positive |
Risks, benefits, and limitations of genetic testing |
Risks, benefits, and limitations of cancer surveillance and risk-reduction options |
Risk of a de novo mutation in the absence of family history |
Risk of receiving an unclear or inconclusive test result |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree