Genetic Syndromes Associated With Increased Risk of Breast Carcinoma | 9 |
Jessica Scott and Carolyn Rogers
HEREDITARY BREAST CANCER—GENERAL CHARACTERISTICS
• The majority of breast cancers, an estimated 90%, are sporadic and result from a combination of risk factors including environmental, hormonal, and stochastic events and are often related to natural aging. However, an estimated 10% of breast cancers are caused by underlying inherited cancer predisposition syndromes (1).
• Dr. Alfred Knudson proposed that hereditary cancer syndromes result from a “two-hit” process (2). In order for a tumor to develop, both copies of the same tumor suppressor gene must be inactivated by mutation. In sporadic cancers, this is caused by two separate sporadic events throughout a person’s lifetime and results in the more common later-onset disease. However, in the case of an inherited cancer predisposition syndrome, a person is born with a germline mutation of one copy of the tumor suppressor gene, allowing for tumor development after only one sporadic event. This results in earlier ages of cancer onset and a significantly increased frequency of disease.
• Of the 10% of breast cancers that are due to an inherited cause, approximately half to two thirds are due to mutations within the BRCA1 and BRCA2 genes (3,4). The remainder are caused by mutations in various other high- and moderate-risk genes (5,6). Additional hereditary breast cancer syndromes may exist that have yet to be identified at this time.
BRCA1 AND BRCA2
Genetics
• The BRCA1 gene is located at 17q21.31 and BRCA2 is found at 13q31.1 (7,8). Mutations in the BRCA genes cause the hereditary breast/ovarian cancer syndrome (HBOC), which is inherited in an autosomal dominant fashion with reduced penetrance (9). These two genes are known to function in the DNA repair pathway by repairing double strand breaks and initiating homologous recombination. Loss of function germline mutations in these genes result in an inherited predisposition to cancer development because of increased genomic instability.
• BRCA gene mutations have been reported in all populations and are predicted to occur at a rate of approximately 1 in 400 people in the general population (9). However, certain populations are known to have higher BRCA mutation frequencies. Most notably are three mutations, two in the BRCA1 gene and one in the BRCA2 gene, which occur at a combined rate of 1 in 40 within the Ashkenazi Jewish population (9,10), placing this population at a significantly increased risk for HBOC. Increased prevalence of HBOC has also been reported in the Icelandic and Dutch populations (9).
Features
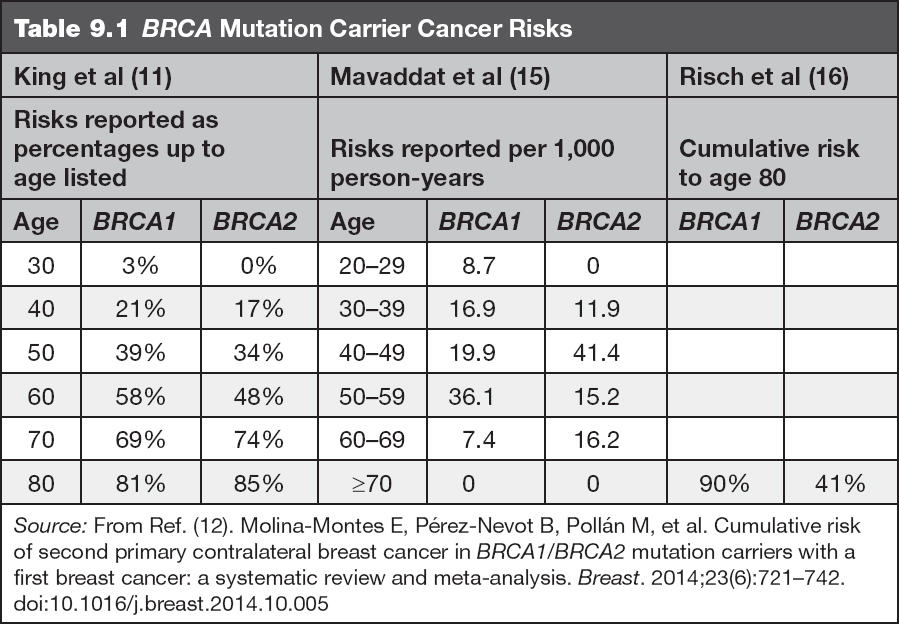
• The lifetime risk for breast cancer (including invasive breast cancers and ductal carcinoma in situ [DCIS]) in female BRCA gene mutation carriers is significantly increased above that of the general population risk of approximately 12%, and is commonly early onset, defined as diagnosis before age 50 years (9). Mutations in BRCA1 confer approximately a 50% to 80% risk for breast cancer for a woman’s lifetime (9,11). In addition, the risk for a second breast primary is 6% to 17% within the first 5 years after the initial diagnosis, and 11% to 31% within the first 10 years after initial diagnosis (12,13). Mutations in BRCA2 result in a lifetime breast cancer risk between 40% and 70%. The risk for contralateral cancer in the first 5 years is between 5% and 15%, and in the first 10 years is between 10% and 29% (12,13). Risk for contralateral cancer with a mutation in either gene reaches 50% to 60% over the woman’s lifetime and varies based on initial age at diagnosis (14). Table 9.1 shows cancer risks by decade, which are separated by gene for quick reference.
• Breast cancers that develop in BRCA mutation carriers are most often invasive ductal adenocarcinoma with an aggressive phenotype, poorly differentiated (grade 3) with a high mitotic rate (17). Additionally, triple-negative (estrogen receptor [ER], progesterone receptor [PR], and HER2 neu negative) breast cancers have been shown to occur more frequently in BRCA1 mutation carriers (17,18).
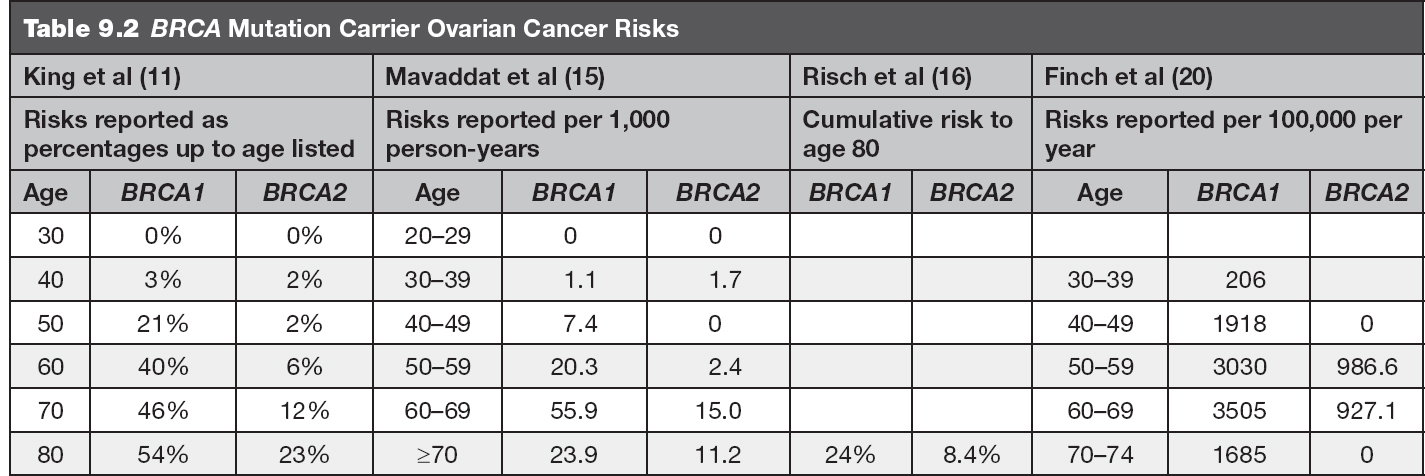
• The lifetime risk for the development of ovarian cancer is estimated to be 39% to 54% for BRCA1 mutation carriers and up to 23% for BRCA2 mutation carriers (11,19). Table 9.2 includes ovarian cancer risks by decade for reference. This cancer risk includes epithelial ovarian cancer, fallopian tube cancer, and primary peritoneal cancer (9).
• Other associated cancers include male breast cancer (1%–2% lifetime risk for BRCA1 carriers and 5%–10% lifetime risk for BRCA2 carriers) and prostate cancer (30% in BRCA1 carriers and up to 39% in BRCA2 carriers) in male carriers as well as melanoma and pancreatic cancer (lifetime risk 1%–3% in BRCA1 carriers and 2%–7% in BRCA2 carriers) (9).
Several studies have been completed to estimate the risks for breast and ovarian cancer in BRCA mutation carriers by age. The risk estimates from several of these articles are included in Tables 9.1 and 9.2.
Medical Management for BRCA Positive Patients
• We generally adhere to NCCN guidelines in our medical management recommendations of BRCA positive patients and patients with other mutations (21):
![]() Regarding breast cancer risk, several options for women who are known to carry deleterious BRCA gene mutations are available and include increased breast cancer surveillance by annual digital mammography and contrast enhanced breast MRI screening beginning at age 25 years, use of chemopreventive agents, or risk-reducing mastectomy.
Regarding breast cancer risk, several options for women who are known to carry deleterious BRCA gene mutations are available and include increased breast cancer surveillance by annual digital mammography and contrast enhanced breast MRI screening beginning at age 25 years, use of chemopreventive agents, or risk-reducing mastectomy.
![]() Regarding the ovarian cancer risk, we recommend risk reducing bilateral salpingo-oophorectomy, typically between the ages of 35 and 40 years and after childbearing. Until the time of completion of oophorectomy, consideration could be given to screening; however, the efficacy of transvaginal ultrasound (US) and CA-125 testing in the early detection of ovarian cancer has not been proven.
Regarding the ovarian cancer risk, we recommend risk reducing bilateral salpingo-oophorectomy, typically between the ages of 35 and 40 years and after childbearing. Until the time of completion of oophorectomy, consideration could be given to screening; however, the efficacy of transvaginal ultrasound (US) and CA-125 testing in the early detection of ovarian cancer has not been proven.
![]() We believe that in addition to annual GYN follow-up with pelvic examination, it is reasonable to offer CA-125 testing annually with completion of transvaginal US, if recommended by the GYN based on the pelvic exam findings or if any other clinical symptoms are noted.
We believe that in addition to annual GYN follow-up with pelvic examination, it is reasonable to offer CA-125 testing annually with completion of transvaginal US, if recommended by the GYN based on the pelvic exam findings or if any other clinical symptoms are noted.
![]() For male carriers, we recommend annual clinical breast exam starting at age 35 years. Annual prostate cancer screening is recommended for all male BRCA2 mutation carriers beginning at age 40 years with consideration of this screening for all male BRCA1 mutation carriers.
For male carriers, we recommend annual clinical breast exam starting at age 35 years. Annual prostate cancer screening is recommended for all male BRCA2 mutation carriers beginning at age 40 years with consideration of this screening for all male BRCA1 mutation carriers.
OTHER HEREDITARY BREAST CANCER SYNDROMES
In addition to the BRCA genes, there are three other genes currently known that are considered to cause high-risk breast cancer syndromes. These genes are CDH1, PTEN, and TP53.
Hereditary Diffuse Gastric Cancer Syndrome
• The hereditary diffuse gastric cancer syndrome (HDGC) is an autosomal dominant condition caused by mutations in the CDH1 gene. This syndrome is characterized by an 80% lifetime risk for the development of diffuse gastric cancer and an approximately 40% to 50% risk for lobular breast cancer in female mutation carriers (22).
![]() Genetics—The CDH1 gene is located at 16q22.1 and codes for epithelial (e-) cadherin, which is a member of a large family of transmembrane proteins (23). These proteins mediate cell–cell adhesion in a Ca2+ dependent manner and play a key role in organ development (24). Loss of functional e-cadherin results in a phenomenon known as cadherin switching and a resultant upregulation of N-cadherin production. N-cadherin upregulation initiates a protein cascade that promotes cellular survival, migration, and invasion, which drives cancer development (24).
Genetics—The CDH1 gene is located at 16q22.1 and codes for epithelial (e-) cadherin, which is a member of a large family of transmembrane proteins (23). These proteins mediate cell–cell adhesion in a Ca2+ dependent manner and play a key role in organ development (24). Loss of functional e-cadherin results in a phenomenon known as cadherin switching and a resultant upregulation of N-cadherin production. N-cadherin upregulation initiates a protein cascade that promotes cellular survival, migration, and invasion, which drives cancer development (24).
Cowden Syndrome
• Cowden syndrome, caused by mutations in the PTEN gene, is associated with an increased risk for breast, nonmedullary thyroid, and uterine cancers as well as various other noncancerous features. Other characteristics include benign disease such as fibrocystic breast disease, thyroid nodules, and leiomyomas, certain dermatological findings including facial trichilemmomas and papules, as well as subcutaneous lipomas, progressive macrocephaly, and, more rarely, learning disabilities (25).
![]() Genetics—The PTEN gene is located at 10q23.31 and functions as a classic tumor suppressor (26). PTEN is involved in numerous functional processes in the body, the most salient being in regulation of the phosphoinositide 3-kinase (PI3K) pathway. In this pathway, PTEN negatively regulates PI3K and results in a decrease in cell cycle progression, induction of cell death, transcription, translation, stimulation of angiogenesis, and stem cell self-renewal. When PTEN function is lost, these cellular processes become less well regulated and can lead to cancer development.
Genetics—The PTEN gene is located at 10q23.31 and functions as a classic tumor suppressor (26). PTEN is involved in numerous functional processes in the body, the most salient being in regulation of the phosphoinositide 3-kinase (PI3K) pathway. In this pathway, PTEN negatively regulates PI3K and results in a decrease in cell cycle progression, induction of cell death, transcription, translation, stimulation of angiogenesis, and stem cell self-renewal. When PTEN function is lost, these cellular processes become less well regulated and can lead to cancer development.
Li–Fraumeni Syndrome
• Li–Fraumeni syndrome (LFS) is an autosomal dominant condition caused by mutations in the TP53 gene that is characterized by a significantly increased risk for the development of breast cancer (potentially with very early onset), leukemia, bone and soft-tissue sarcomas, and brain tumors. Adrenocortical carcinoma and choroid plexus tumors are considered highly suggestive of LFS (27).
![]() Genetics—The TP53 gene is located at 17p13.1 and is involved in cell cycle regulation including inducing apoptosis or cell cycle arrest. Germline mutations in this gene result in LFS; however, somatic loss of p53 protein expression is almost universal in human cancer development regardless of the presence or absence of an underlying hereditary cancer syndrome (28).
Genetics—The TP53 gene is located at 17p13.1 and is involved in cell cycle regulation including inducing apoptosis or cell cycle arrest. Germline mutations in this gene result in LFS; however, somatic loss of p53 protein expression is almost universal in human cancer development regardless of the presence or absence of an underlying hereditary cancer syndrome (28).
In addition to these high-risk breast cancer syndromes, there are multiple known moderate-risk genes for which clinical genetic testing is also available. Of the known moderate-risk genes, only a portion currently have medical management guidelines published. The National Comprehensive Cancer Network (NCCN) currently recommends that carriers of mutations in various genes, including but not limited to the ATM, CHEK2, PALB2, and STK11 genes, undergo increased breast cancer surveillance by annual mammography and breast MRI (21). Additionally, consideration could be given to risk-reducing bilateral mastectomy for PALB2 mutation carriers but is not currently recommended for ATM, CHEK2, or STK11 mutation carriers as they are understood to have lower breast cancer risks (22). Risk-reducing oophorectomy is currently recommended for BRIP1, RAD51C, and RAD51D mutation carriers in addition to BRCA mutation carriers (22). Other moderate-risk genes have been identified for which there is clinical genetic testing available. For some of these genes, increased surveillance is recommended and for others the medical management recommendations for carriers are still based on the known family history.
INDICATIONS FOR REFERRAL TO GENETIC COUNSELING AND TESTING
• Genetic counseling is recommended for all patients considering and undergoing genetic testing. The 2003 American Society of Clinical Oncology (ASCO) policy statement “strongly recommend(s) that genetic testing be done only in the setting of pre- and posttest counseling, which should include discussion of possible risks and benefits of cancer early detection and prevention modalities” (29). Therefore, referral for genetic counseling is critical in advance of testing to ensure the most appropriate testing is ordered and that full informed consent is obtained from the patient.
![]() Patients who have completed genetic testing, with or without the benefit of pretest genetic counseling, and are found to carry a mutation in a cancer susceptibility gene should be provided in-depth posttest genetic counseling to discuss the implications of the mutation for their own medical management and to identify at-risk family members.
Patients who have completed genetic testing, with or without the benefit of pretest genetic counseling, and are found to carry a mutation in a cancer susceptibility gene should be provided in-depth posttest genetic counseling to discuss the implications of the mutation for their own medical management and to identify at-risk family members.
![]() Posttest genetic counseling is of benefit to all patients undergoing testing to ensure accurate interpretation of results.
Posttest genetic counseling is of benefit to all patients undergoing testing to ensure accurate interpretation of results.
The NCCN Practice Guidelines in Oncology “Genetic/Familial High-Risk Assessment: Breast and Ovarian Cancer” provide the following indications for genetic risk evaluation (21):
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


