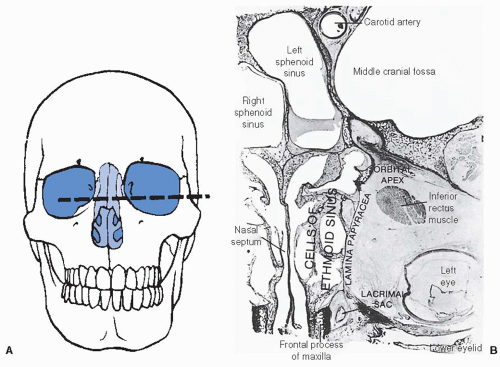
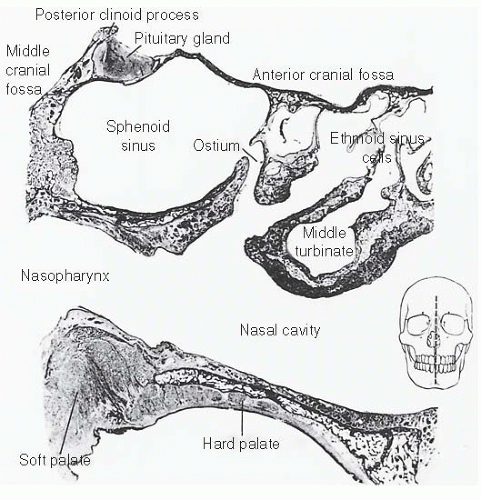
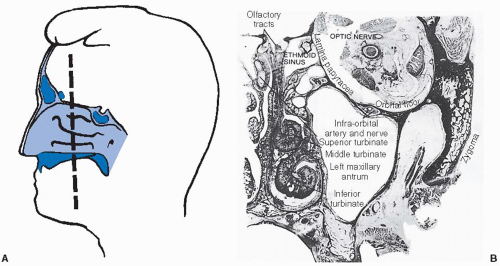
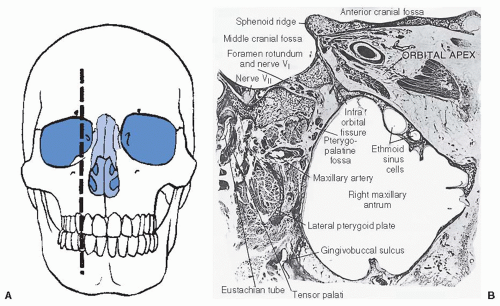
further because these patients have a history of nasal polyps, which produce similar symptoms. Tumors of the maxillary antrum also tend to remain silent until they extend outside the sinus. As a result of tumor extension to the premaxillary region, patients can complain of facial pain, numbness, localized swelling, nasal obstruction, and epistaxis. Nasal obstruction from tumor extension to the nasal cavity and tumor extension into the orbit causing ocular problems such as epiphora, proptosis, diplopia, eye pain, and inner canthal mass are the most common symptoms of ethmoid sinus tumors. Sixth nerve palsy, severe morning headaches, and diplopia are common symptoms for primary sphenoidal tumors. Lastly, frontal sinus tumors can present with bilateral swelling of the glabella and the supraorbital ridge and with frontal pain.
TABLE 21.1 Seventh Edition of AJCC Staging for Nasal Cavity and Paranasal Sinus Cancer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
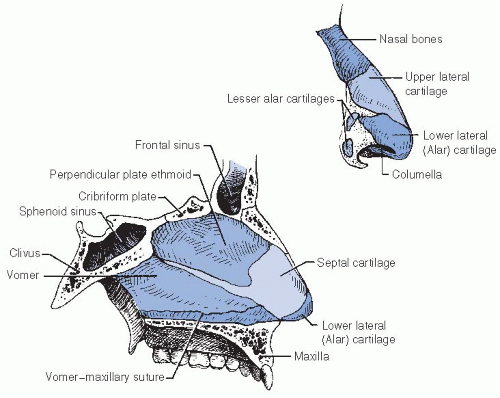
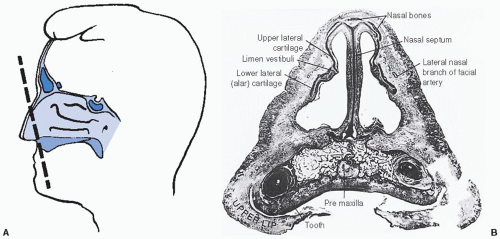
the olfactory nerve, which innervates the superior nasal concha and upper third of the septum.
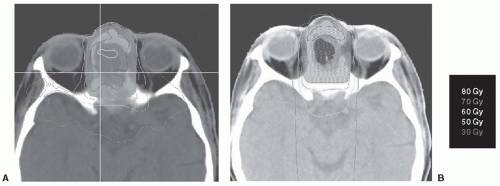 FIGURE 21-1. An example of an ethmoid sinus tumor being planned using (A) IMRT and (B) 3D-CRT. Notice that the 70-Gy isodose line (dark blue) coverage is superior in the IMRT versus the 3D-CRT plan. In addition, there is superior sparing of the optic chiasm when compared with the 3D-CRT plan. IMRT, intensity modulated radiation therapy; 3D-CRT, three-dimensional conformal radiotherapy. (See color insert.) |
TABLE 21.2 Kadish Staging of Esthesioneuroblastoma | ||||||
|---|---|---|---|---|---|---|
|
of proper sites to be irradiated must be considered. For example, for patients with tumors at risk for perineural spread such as adenoid cystic carcinomas, the planned target volume should include the neural pathways up to the cranial nerve ganglion at the base of skull.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree