General Principles and Management
Bruce J. Davidson
Kenneth A. Newkirk
Kenneth D. Burman
THYROID CANCER
Introduction
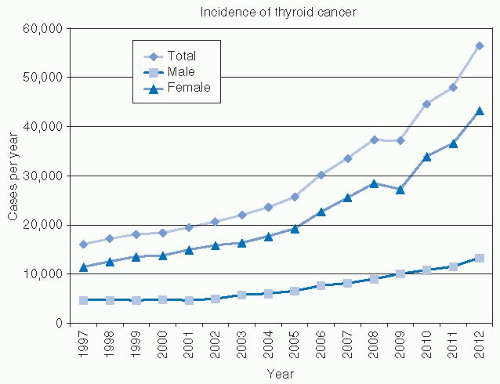
Epidemiology—Increasing Incidence of Thyroid Cancer. According to cancer statistics, there has been a substantial rise in thyroid carcinoma in the United States in recent years. Cancer statistics for 2012 estimate 56,460 new cases of thyroid cancer will occur. This is more than three times the number of thyroid cases reported 15 years earlier (Fig. 28-1).1 The mortality is increasing at a slower rate, with 1,780 deaths expected due to thyroid cancer in 2012.1 Were thyroid cancer outcomes more ominous, the public awareness of this significant trend would be widespread. In fact, the rise in incidence is of uncertain significance. Although this increased incidence may represent a true increase in disease, there are other possible explanations including reporting variability, increased diagnostic effort, and improved technology.
Although the reported incidence of thyroid cancer is alarming, these figures must be assessed in the context of data implying a much larger prevalence of these tumors. Several autopsy series around the world have suggested that the prevalence of occult papillary cancer (typically defined as tumors <1 cm in diameter) in patients dying of other causes ranges from 6% to 28%.2 In the United States, studies have shown the incidence of occult papillary cancer to be 6% to 13% in patients dying of other causes.2,3,4 If one were to make a very conservative estimate of occult thyroid cancer in the United States at 1 % of adults, it could be estimated that there are currently >3 million undiagnosed thyroid cancers. The Surveillance, Epidemiology, and End Results (SEER) database estimated a prevalence of thyroid cancer of 458,403 in 2010; therefore, it would appear that what is seen is only a minority of the total number of thyroid cancers in cancer statistical data.5
There are several suspected reasons for the increased incidence of thyroid cancer. These reasons include (a) an increased reporting of occult tumors, (b) an increased diagnostic suspicion due to increased awareness by clinicians, (c) an improvement in diagnostic skills through increased ultrasound surveillance, (d) an increased use of fine-needle aspiration (FNA), and (e) the growing use of ultrasound-guided FNA to evaluate small lesions of the thyroid. If any or all of these reasons were the cause of the increase in thyroid cancer incidence, one might expect that there would be an increase in the proportion of cancers diagnosed at lower stage.
Using SEER data from 1975 to 2002 and historical staging, there has been no change in the proportion of thyroid cancer that is confined to the thyroid, so it would not appear that increases are of low-stage tumors only.6 However, improvements to the SEER database beginning in 1988 lend further insight. First, it has been shown that the entire increase in incidence of thyroid cancer consists of papillary carcinomas. Secondly, as primary size has been better reported by SEER since 1988, 49% of the increased incidence between 1988 and 2002 consists of tumors <1 cm diameter, and 87% of the increase consists of tumors <2 cm.6 Therefore, a significant portion of this “epidemic” of thyroid cancer would appear to be a reflection of increased investigation and assessment of small thyroid lesions. The controversy about this is not resolved as more recent analysis of SEER data shows that although the majority of the increase in thyroid cancer is among low-stage tumors, a measurable increased incidence can be shown for higher stage tumors as well.7
Although the diagnosis of small papillary cancers appears to be responsible for the majority of increase in thyroid cancer incidence, it is difficult to predict the natural history of even small papillary thyroid cancers and clinical judgment is important in the monitoring of such patients.8 Mazzaferri has cautioned that even patients with micropapillary cancer have a cancer-related mortality of approximately 1%, a risk of distant recurrence of approximately 2.5%, and a risk of local node recurrences of approximately 5.0%. These figures are why clinicians are uneasy as they try to address this epidemic.
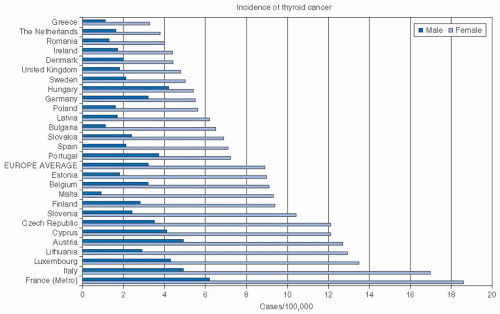
Worldwide Epidemiology. The worldwide incidence of thyroid cancer varies as shown in Figure 28-2, with the highest reported incidence in Polynesia, followed by North America. In general, the
incidence in more developed countries is higher than that in less developed countries.9 Worldwide incidence varies considerably, and even within Europe (Fig. 28-3), wide variations are seen.10
incidence in more developed countries is higher than that in less developed countries.9 Worldwide incidence varies considerably, and even within Europe (Fig. 28-3), wide variations are seen.10
With such a wide variation in incidence of thyroid cancer, the impact of environmental exposures may be considered. For the countries surrounding the Chernobyl nuclear facility, an increase in thyroid cancer incidence has been shown and more will be written on this later in the chapter.11 However, similar to the U.S. experience, multiple studies have demonstrated an increase in thyroid cancer incidence throughout the world. A relationship to the Chernobyl incident or to other radiation or environmental exposures has not been suspected in this increase.12 Instead, the increase in thyroid cancers has been explained by a changing management strategy in thyroid disease. A French multi-institutional study demonstrates a relationship to both the increased use of diagnostic ultrasound and to rigorous cytologic evaluations. The result has been an increase in the proportion of surgical cases that result in malignant pathology.13
Lithuania introduced ultrasound-guided FNA in 1997 and a recent retrospective evaluation shows a significant increase in the incidence of thyroid cancer beginning in 2000. Before this, the annual increase in thyroid cancer incidence was approximately 5 % per year. Since 2000, the annual increase in thyroid cancer is 28 % per year. The increased incidence is seen in both papillary and follicular cancers, but is confined to stage I disease only.12
Changes in Histology. Over the last 50 years, there has been an increase in the frequency of papillary cancers primarily, with a decrease in follicular cancers and anaplastic cancers and essentially no change in medullary cancers.14 It has been proposed that the difference in the proportion of tumors of each histology is partly explained by variations in environmental iodine exposure. Iodine-deficient areas are associated with an increased risk of goiter and an increased proportion of follicular cancer and anaplastic cancer. Iodine-sufficient areas are associated with increased autoimmune thyroid disease and an increased proportion of papillary thyroid cancers with a decrease in follicular and anaplastic cancers. A dose-response relationship is indicated with the proportion of papillary to follicular cancers changing from approximately 5:1 in high iodine-intake areas, to 2.5:1 in moderate iodine areas to 1:1 in iodine-deficient areas.15
Changes in pathologic interpretation of thyroid cancers over time may confound our ability to make generalizations about the proportion of cancer histology over time and about the relationship between iodine and thyroid cancer histology. A review of previous thyroid cancer cases entered in the Geneva Cancer Registry over a 30-year period demonstrated that 45 % of follicular cancers diagnosed between 1970 and 1979 were reclassified as papillary cancers. Cases entered more recently, 1990 to 1998, were less likely to be reclassified, but the rate of reclassification was still 25 %. The possible explanation for this high rate of reclassification is not known but could relate to the development of stricter guidelines for microscopic characterization.16
Etiology. A relationship between ionizing radiation and thyroid cancer was first described in 1950 by Duffy and Fitzgerald.17 A dose-response relationship has been established for lower doses of radiotherapy, particularly in individuals exposed in childhood.18 Between approximately 1920 and 1960, the use of low-dose irradiation was practiced for benign diseases of childhood
including acne, tinea capitis, impetigo, sinusitis, adenoid hypertrophy, thymic enlargement, and keloids. The practice has now been discontinued since the recognition that this practice contributed to an increased risk papillary thyroid cancer development. However, the risk of developing thyroid cancer in patients treated with low-dose radiotherapy is increased, even for doses as small as 0.1 Gy, and although the risk begins to decline at 30 years, it remains elevated far longer. The risk is greatest for those irradiated at younger than 15 years of age with an estimated relative risk of 7.7 per Gy.18 The risk of nodules in patients irradiated for benign disease has been reported at 38%19 and approximately a third of these nodules will be malignant.20,21,22 While the risk is significant for those patients irradiated, the numbers of irradiated patients is not felt to be large enough to explain the increase in cancer incidence across populations discussed earlier.
including acne, tinea capitis, impetigo, sinusitis, adenoid hypertrophy, thymic enlargement, and keloids. The practice has now been discontinued since the recognition that this practice contributed to an increased risk papillary thyroid cancer development. However, the risk of developing thyroid cancer in patients treated with low-dose radiotherapy is increased, even for doses as small as 0.1 Gy, and although the risk begins to decline at 30 years, it remains elevated far longer. The risk is greatest for those irradiated at younger than 15 years of age with an estimated relative risk of 7.7 per Gy.18 The risk of nodules in patients irradiated for benign disease has been reported at 38%19 and approximately a third of these nodules will be malignant.20,21,22 While the risk is significant for those patients irradiated, the numbers of irradiated patients is not felt to be large enough to explain the increase in cancer incidence across populations discussed earlier.
While childhood radiation for benign conditions is no longer used, there remains a cohort of patients who are treated in childhood for malignancy with radiation therapy. This is a population that has been shown to be at increased risk of thyroid cancer. Estimates of increased risk ratio (RR) of 4.6 to 14.6 have been reported.23,24 The risk increases with increasing thyroid radiation dose up to about 20 Gy and then tapers off.24 The risk is greater with younger age of treatment and in females. Subsequent thyroid cancer risk is most often associated with Hodgkin and non-Hodgkin Lymphoma23 and neuroblastoma.24 For lymphomas, the field of radiation includes the thyroid bed whereas for the latter tumor the early age of treatment is a major factor influencing thyroid cancer risk. The relative risk of developing thyroid cancer peaks at 20 years after treatment,23,24 but lifelong monitoring is recommended.23
There has been evidence of increased rates of differentiated thyroid cancer (DTC) in areas downwind from nuclear test sites.24 Following atomic testing in the United States between 1950 and 1958, the estimated average cumulative dose of radiation from environmental iodine-131 ( 131I) has been estimated to range from 1 to 4 rads per individual across the United States. Some areas are estimated to have had exposures up to 16 rads. In contrast, the average annual background radiation is 0.1 rad.26 A careful evaluation of thyroid cancer rates attributable to atmospheric nuclear testing found that the risk of thyroid cancer was only suggested for individuals exposed before the age of 1. Older children and adults did not demonstrate an increased risk.26
The risk of environmental exposure from radiation has been clearly documented following the Chernobyl nuclear disaster in the Ukraine in 1986.27,28,29 The pattern of development, however, is quite different from that seen in childhood therapeutic radiation exposure. In childhood low-dose therapeutic radiation exposure, the risk of thyroid cancer rises after a latency of approximately 10 years and peaks at 25 to 30 years as shown in Figure 28-4.30 Following Chernobyl, the increase in thyroid cancers was seen as early as 1989, only 3 years after the accident.27,28,29 The impact of the Chernobyl disaster may not be yet fully known. Data from Belarus (whose border is <20 miles from Chernobyl) indicate a significantly higher incidence of thyroid cancer in high-exposure areas when compared with low-exposure areas. The rate of thyroid cancers continues to increase in individuals who were between 0 and 15 years of age at the time of the disaster.11
Other environmental factors that might lead to an increase in thyroid cancer development are not clear. In a thorough case-control study using the Swedish Cancer Registry and an extensive review of lifetime diagnostic radiograph exposures, there was no evidence of an increased exposure to diagnostic radiographs in patients with DTC.31 Similarly, there is no strong indication that irradiation from the use of therapeutic 131I leads to an increased risk of thyroid cancer.32,33,34,35 One exception is work by Franklyn et al. which evaluated patients with hyperthyroidism given 131I and found a higher incidence and mortality rate of thyroid cancers as well as a higher incidence of small bowel tumors, although the absolute incidence rates were low.36
Associations between thyroid cancer and other exposures have been investigated. Owing to the fact that thyroid cancer is a relatively common disease in young women and the increased incidence of thyroid cancer is greatest in women, multiple studies have investigated the relationship between thyroid cancer and reproductive history and exogenous hormone use. The data are mixed but describe an increased risk of thyroid cancer with early menarche,37 early first pregnancy,38 late first pregnancy,39 and late last pregnancy.39,40 Other studies show no relationship to reproductive history.41,42 The relationship between oral contraceptive use and thyroid cancer has been positive, negative, or unrelated depending on the study,37,43,44 although there is a suggestion from pooled data that oral contraceptives can act as a promoter of thyroid cancer.43 The risk of oral contraceptive use appears to be eliminated after the hormone is discontinued.43
Genetic causes of thyroid malignancy are well known for medullary cancer, and this will be discussed in detail later in the chapter. For papillary cancer, there are a handful of syndromes that manifest this cancer. These include Gardner syndrome, Cowden disease, and Carney complex, all autosomal dominantly inherited.45,46
Relevant Anatomy
Embryology. The thyroid gland develops from the endodermal tissues of the primitive gastrointestinal tract. The site of origin ultimately is the foramen cecum at the tongue base and descent of the thyroid occurs by the seventh gestational week to lie anterior to the cricoid and cervical trachea. The thyroid is able to concentrate iodine and form thyroid hormone by the 11th gestational week. It reaches its full size of 15 to 25 g in adulthood.
The descent of the thyroid through the anterior midline neck explains several anomalies that relate to thyroid pathology. These include ectopic thyroid located at the tongue base, termed a lingual thyroid. Lingual thyroid tissue has been reported in up to 10% of children.47,48 When noted, this may represent the only thyroid tissue in patients and evaluation of the neck for additional thyroid tissue is important. Along the pathway of thyroid descent, a cyst of ciliated pseudostratified epithelium and variable amounts of thyroid tissue may remain. These thyroglossal duct cysts are usually closely related to the central portion of the hyoid bone, and it is generally accepted that this bone must be resected to successfully remove the cyst. The cyst, or similarly located ectopic thyroid tissue, may give rise to thyroid neoplasia
on occasion. The most inferior point on the pathway of normal thyroid descent would be represented by the pyramidal lobe, thyroid tissue located just cephalad to the thyroid isthmus.
on occasion. The most inferior point on the pathway of normal thyroid descent would be represented by the pyramidal lobe, thyroid tissue located just cephalad to the thyroid isthmus.
Other ectopic locations of thyroid tissue have been noted including mediastinum, heart, liver, esophagus, larynx and trachea, and ovary.49,50,51,52,53,54 These structures are obviously beyond the range of the typical descent of thyroid tissue and represent true ectopic locations. Thyroid rests in cervical lymph nodes deserve special mention. Although these have been described previously in cervical lymph nodes,55 the concern when thyroid tissue is identified in these nodes is that this represents well-DTC metastatic to that node. As a general rule, thyroid rests in nodes along the jugular chain (i.e., lateral neck nodes) are considered diagnostic for thyroid malignancy. On the other hand, thyroid rests in central compartment lymph nodes are felt to represent benign thyroid rests if no suspicion of malignancy is noted in the thyroid itself. These guidelines may not apply to all patients and each patient’s context should be reviewed to determine when additional testing for the presence of malignancy must proceed.
While the thyroid follicular tissue develops from the embryologic foregut, the calcitonin producing (C or parafollicular) cells of the thyroid develop from neural crest ectoderm related to the fourth and fifth branchial pouches. These tissues, known as the ultimobranchial body, migrate anteriorly and merge with the descending follicular thyroid tissues, thus explaining their designation as parafollicular cells. Analysis of thyroid tissues has shown that these C cells lie in greater concentration in the superior and posterior aspect of the thyroid, a location similar to the superior parathyroid, which also arises from the fourth brachial pouch.
The parathyroid glands develop as two paired structures from the third and fourth branchial pouches. The third pouch gives rise to the inferior parathyroid and the more inferior thymus gland. This relationship explains the fact that ectopic parathyroid tissues may be located within the thymus. As stated, the superior parathyroid develops from the fourth branchial pouch along with the ultimobranchial body, which gives rise to the C cells.
Anatomy
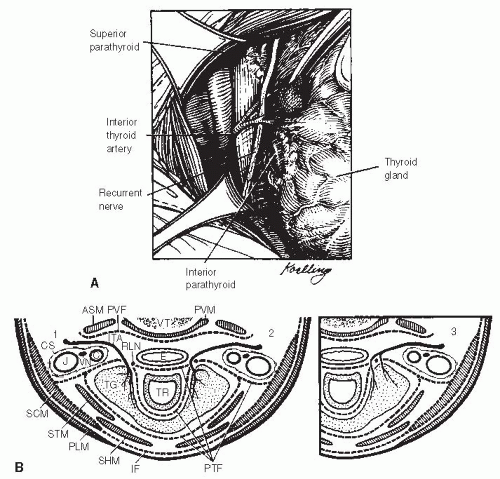
The thyroid consists of two lobes, each approximately 8 to 10 mL in size connected by a thin isthmus lying over the trachea. A pyramidal lobe of any significant size is present in a minority of cases. The average gland weighs 15 to 25 g. The thyroid isthmus is often easily palpated, just below the cricoid cartilage. The thyroid lobes are palpable lateral to this, with the examination made easier when the patient swallows. The left and right lobes of the thyroid lie just deep to the sternothyroid muscles. The gland is encased by a thin layer of fascia, but the posterior attachment of the gland to the trachea is quite dense and lies just medial to the area where the recurrent laryngeal nerve enters the cricothyroid membrane. It has been estimated that up to two-thirds of thyroid glands may have a tubercle of Zuckerkandl, a posterior, lateral extension of the thyroid lobe that may be an important landmark in identifying the recurrent laryngeal nerve and the superior parathyroid gland.56,57,58
Vascular and Lymphatic Anatomy. The vascular anatomy of the thyroid is described as two paired arteries and three paired veins. These include superior and inferior thyroid arteries and superior, middle, and inferior thyroid veins. Although this is true, the practical application of this information is significantly limited by the surgical anatomy of the thyroid. The superior thyroid vein, and to a lesser extent the artery, anastomose with the thyroid through several small branches. The superior laryngeal nerve may be intertwined with these branches, so meticulous dissection of individual branches of the superior thyroid artery and vein is required when dissecting the superior pole of the thyroid. Likewise, the inferior vein is always divided into multiple branches at the margin of the thyroid gland. Individual ligation of these branches is required due to the fact that the inferior parathyroid is often located on the thyroid capsule in this area. Finally, the inferior thyroid artery has been shown to contribute the major blood supply to both the inferior and superior parathyroid glands. Preservation of the vascularity of these glands is felt to be important in order to preserve their function after thyroidectomy. Therefore, dissection and ligation of only the distal branches of this artery as they enter the thyroid has been felt to be most judicious. Regarding the last point, however, there may be some debate as two randomized studies looking at subtotal thyroidectomy found no difference in postoperative hypocalcemia whether or not the inferior arteries were ligated at the main trunk.59,60 Whether these studies apply to the performance of total thyroidectomy is unclear.
The superior thyroid artery arises as the first branch of the external carotid artery in the vast majority of individuals. From this point, it descends to the upper thyroid pole by passing deep to the omohyoid muscle and superficial to the superior constrictor muscle and cricothyroid muscle. The superior laryngeal nerve arises from the vagus nerve high in the neck and passes deep to the carotid artery to reach the larynx. The internal branch of the superior laryngeal nerve enters the thyrohyoid membrane just inferior to the hyoid whereas the external branch travels parallel and deep to the superior thyroid artery and then passes medially to the cricothyroid muscle.
The relationship between the superior thyroid artery and nerve is variable. This variability was emphasized in a recent anatomic study that included review of previous data. In this, the nerve was shown to cross the plane of the superior thyroid artery over 1 cm above the superior thyroid pole in 18% to 68% of cases. The nerve crossed within 1 cm of the superior thyroid pole in 11% to 60%. However, the more important, and most consistent, anatomic relationship for surgical dissection was the finding that the nerve crosses the artery below the upper pole of the thyroid in 14% to 20% of patients.61 In addition to this study, recent work has shown that in almost half of all thyroidectomies the superior laryngeal nerve may be located below the superior pole of the thyroid gland and be at risk for injury,62 with oversized glands having a higher risk.63
The inferior thyroid artery is a branch of the thyrocervical trunk and passes deep to the common carotid artery to reach the thyroid. In this orientation, it is roughly perpendicular to the recurrent laryngeal nerve. The relationship between the inferior thyroid artery and this nerve is variable, but is stated to demonstrate the nerve as deep to the artery in 70% of cases. A distinction has been suggested between the two sides of the larynx with the right nerve most often passing between branches of the inferior thyroid artery and the left nerve most often passing behind the artery.64
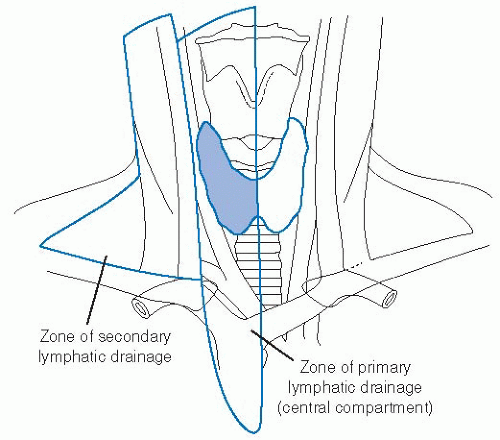
Lymphatics of the thyroid consist of a rich intraglandular network as well as extraglandular drainage. The intraglandular network has been thought to explain the high rate of multifocality in papillary thyroid cancers by allowing tumor emboli to travel through the thyroid and metastasize to other intrathyroidal sites. The extraglandular network passes through the medial compartment of the neck and also follows the arterial supply in a retrograde manner as it exits this medial compartment. The medial or central compartment of the neck, shown in Figure 28-5, represents the primary echelon nodal drainage from the thyroid gland. Surgical dissection of the central compartment in the treatment of thyroid papillary carcinoma has been shown to yield an average of eight nodes per patient.65
Secondary echelon nodal drainage is to the lateral neck compartment and posterior triangle lymph nodes. The retrograde lymphatic drainage following the arterial supply to the thyroid
is demonstrated by work on subcentimeter papillary thyroid cancers. Small tumors of the upper portion of the thyroid are as likely to spread to level II in the upper jugular chain (presumably through lymphatics along the superior thyroid artery) as to lower neck nodes.66
is demonstrated by work on subcentimeter papillary thyroid cancers. Small tumors of the upper portion of the thyroid are as likely to spread to level II in the upper jugular chain (presumably through lymphatics along the superior thyroid artery) as to lower neck nodes.66
Neuroanatomy. The nerves important in thyroid anatomy and thyroid surgery are those of the larynx. The superior laryngeal nerve and recurrent laryngeal nerve are branches of the vagus nerve. This nerve exits the skull base at the jugular foramen, and just below this point, the superior laryngeal nerve branches off to pass deep to the carotid artery and medially toward the larynx. The internal branch of this nerve enters the thyrohyoid membrane to supply sensory innervation to the supraglottic larynx. The superior location of this branch means that is it not at risk in thyroid surgery unless a large cephalad extension of the thyroid is present. The external branch of the nerve is of greater concern in thyroid surgery. This branch innervates the cricothyroid muscle, a nerve important in vocal fold stretch and in vocalizing at high pitch. The nerve travels to this muscle along the superior thyroid artery, to which it lies deep. Near the upper pole of the thyroid and occasionally between branches of the superior thyroid artery, the nerve travels medially to the cricothyroid muscle. En route, the nerve may penetrate the inferior constrictor muscle and, in fact, has been shown to travel deep to this muscle throughout its course in 10% of patients.61 The nerve is quite small and even with significant effort can prove difficult to identify. In fact, one study reported an effort to identify the nerve in 50 cases using a nerve stimulator and was only able to identify the nerve in 20% of cases.67 Other surgeons have been more successful and have reported success in identifying the nerve in >90% of cases.68
The recurrent laryngeal nerve branches from the vagus nerve in the thoracic cavity. On the left side, the nerve passes around the aortic arch and then superiorly up along the tracheoesophageal groove to the Berry ligament. On the right side, the nerve passes around the subclavian artery and then passes in a more oblique direction toward the larynx. The nerve passes deep to the inferior thyroid artery in most cases (Fig. 28-6). As the nerve proceeds cephalad, small branches may be seen that enter the
trachea and esophagus. These include sensory branches to the upper trachea and subglottic larynx. The motor component of the recurrent laryngeal nerve may divide into a posterior and anterior division below the level of the Berry ligament. Katz describes branching of the nerve over 5 mm below the lower border of the cricoid cartilage in 63% of >1,100 thyroidectomies.69 In addition, recent work suggests that in 1% of cases the recurrent nerve or its branches may enter the larynx in a higher-thanexpected location making the nerve more prone to injury.70 It is important to recognize this branching of the nerve during thyroid surgery. Failure to recognize this anatomy may result in injury to a branch of the nerve. The function of the motor branches of the recurrent laryngeal nerve is to supply all of the muscles of the larynx except the cricothyroid. These include both the abductors and the adductors of the larynx. Injury to the nerve can result in significant laryngeal incompetence. When disruption of those sensory fibers from the recurrent laryngeal nerve to the upper trachea and subglottic larynx occurs in conjunction with motor injury to the recurrent nerve, the combination of vocal cord incompetence and hemitracheal anesthesia can make the postoperative course of such a patient much more problematic.
trachea and esophagus. These include sensory branches to the upper trachea and subglottic larynx. The motor component of the recurrent laryngeal nerve may divide into a posterior and anterior division below the level of the Berry ligament. Katz describes branching of the nerve over 5 mm below the lower border of the cricoid cartilage in 63% of >1,100 thyroidectomies.69 In addition, recent work suggests that in 1% of cases the recurrent nerve or its branches may enter the larynx in a higher-thanexpected location making the nerve more prone to injury.70 It is important to recognize this branching of the nerve during thyroid surgery. Failure to recognize this anatomy may result in injury to a branch of the nerve. The function of the motor branches of the recurrent laryngeal nerve is to supply all of the muscles of the larynx except the cricothyroid. These include both the abductors and the adductors of the larynx. Injury to the nerve can result in significant laryngeal incompetence. When disruption of those sensory fibers from the recurrent laryngeal nerve to the upper trachea and subglottic larynx occurs in conjunction with motor injury to the recurrent nerve, the combination of vocal cord incompetence and hemitracheal anesthesia can make the postoperative course of such a patient much more problematic.
The anatomy of the recurrent laryngeal nerve results in some specific risks during thyroid surgery. The nerve can be placed under significant stress during the surgery as dissection proceeds along the lateral aspect of the thyroid and the gland is retracted medially to expose the path of the nerve. During this medial retraction of the gland, the nerve may become compressed by a distal branch of the inferior thyroid artery or by a fascial band stretched across the nerve. At the ligament of Berry, the nerve courses posteriorly to enter the larynx behind the cricothyroid joint. This fixed relationship between the nerve and the cricothyroid joint and the medial traction of the gland described earlier can also contribute to stretch on the recurrent laryngeal nerve during the dissection. Inadvertent transection of the recurrent laryngeal nerve is not a significant risk for an experienced thyroid surgeon. However, stretch injuries of the nerve may still occur. Almost all of these are temporary. The use of intraoperative recurrent nerve monitoring has been suggested to decrease the risk of injury. However, in recent review of the literature, Dralle et al.71 found that in six studies comparing intraoperative recurrent nerve monitoring with postoperative laryngoscopic findings, nerve monitoring had a high negative predictive value (NPV; 92%-100%), but relatively low and variable positive predictive values (PPVs; 10%-90%), potentially limiting the role of nerve monitoring. International study guidelines have recently been published on the role of intraoperative nerve monitoring.72
A special anatomic relationship should be mentioned in the case of the right recurrent laryngeal nerve. This nerve may be nonrecurrent in a small number of patients, typically estimated at well below 1 %. This occurs when there is an aberrant takeoff of the right subclavian artery. This anomaly represents an anomaly of the fourth branchial arch resulting in the right subclavian to arise from the aorta and pass deep to the esophagus. The recurrent laryngeal nerve will then pass deep to the carotid and directly toward the larynx. The authors have seen nonrecurrent nerves that travel parallel (and deep) to the superior thyroid artery and others that resemble a recurrent nerve except that they travel behind the carotid at the level of the inferior thyroid artery.
Parathyroid Anatomy. Normal parathyroid glands are each approximately 40 to 70 mg in weight, meaning a size of approximately 4 × 6 mm at surgery. The location of these glands is variable. The typical location of the superior parathyroid gland is behind the thyroid at the cricoid level, lateral to the recurrent laryngeal nerve. The inferior glands are located on or adjacent to the lower pole of the thyroid in 50% of cases. The inferior glands may lie within thymic tissue in up to 40% of cases.
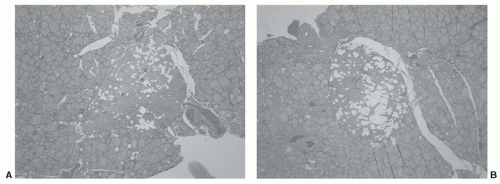
In the treatment of primary hyperparathyroidism, the surgeon must be familiar with various ectopic locations of parathyroid tissue in order to identify and resect adenomas or hyperplastic glands in these locations. The treatment of malignant diseases of the thyroid does not typically require direct application of this knowledge. However, the surgeon should be aware that 4% of parathyroid glands are found embedded within the thyroid gland.73 Figure 28-7 shows such a case where both inferior parathyroid glands were identified within the thyroid on pathologic study. This case emphasizes both the possibility of intrathyroidal parathyroid gland and the symmetry often seen when searching for parathyroid glands at surgery. Beyond identification and preservation of parathyroid glands while dissecting the thyroid gland, the treatment of thyroid malignancy will often require a medial compartment neck dissection. Here, the parathyroid glands are at significantly greater risk of devascularization or inadvertent removal during the dissection process and surgeons must be facile at parathyroid gland identification, dissection, and preservation of the parathyroid vascular pedicle, and when necessary, perform parathyroid autotransplantation, usually into an adjacent muscle.
Physiology
The application of thyroid physiology in the treatment of patients with thyroid cancer is one of the basic tools of the clinician. These applications include the possible use of exogenous thyroid hormone to suppress stimulation of benign and malignant thyroid tissue, withdrawal of thyroid hormone to allow intrinsic stimulation to be maximized in anticipation of radioactive iodine, and, more recently, the use of exogenous thyroid stimulation to prepare patients for radioactive iodine without the need for exogenous thyroid hormone withdrawal.
The basic functional unit of the thyroid is the thyroid follicle, which consists of a space filled with colloid and lined by thyroid follicular cells. These cells avidly trap iodide molecules through sodium iodide symporter channels and transport this iodide to the follicular lumen. Meanwhile, follicular cells also synthesize thyroglobulin (Tg), a glycoprotein that serves as the backbone and storage molecule for thyroid hormone. On the luminal side of the follicular cell plasma membrane, iodide molecules are oxidized and attached to tyrosyl peptides of the Tg molecule. Subsequent coupling of iodinated tyrosyl residues leads to the formation of the functional thyroid hormones, still embedded within the Tg glycoprotein through these tyrosyl residues.74 The two active thyroid hormones, thyroxine (T4) (3,5,3′,5′-tetraiodo-thyronine) and 3,5,3′-triiodothyronine (T3), are stored in this manner within the thyroid colloid until released into circulation. Release of active hormone occurs by reverse pinocytosis from the intrafollicular space back through the follicular cell where the Tg molecule is cleaved to release the T3 and T4 hormones into the plasma.
Tg itself may leak out or be released from follicular cells into the circulation, and the serum measurement of Tg has become an important tumor marker. The molecule is present in serum in normal and pathologic conditions. Increased serum Tg concentrations may be seen in the hyperthyroid phase of silent or subacute thyroiditis; multinodular thyroid disease; and in papillary, follicular, and anaplastic thyroid cancers. The use of serum Tg measures in previously untreated patients (with intact thyroid glands) is limited by its lack of specificity to distinguish benign from malignant conditions. However, in patients previously treated for DTC, in whom there is little or no residual normal thyroid tissue, Tg has become an essential indicator of residual or recurrent DTC (i.e., papillary or follicular thyroid cancer).
In plasma, T3 and T4 are bound to thyroxine-binding globulin (TBG) as well as prealbumin and albumin. Thyroxine-binding globulin is a protein made in the liver and is the dominant thyroid hormone-binding protein. T4 mainly acts as a prohormone and is metabolized to the active form of thyroid hormone, T3, in the liver and kidney primarily. Actions of thyroid hormones influence growth, development, and metabolism. In adulthood, the major effects of thyroid hormone are in the regulation of metabolism, including changes in oxygen consumption and protein, carbohydrate, lipid, and vitamin metabolism.75
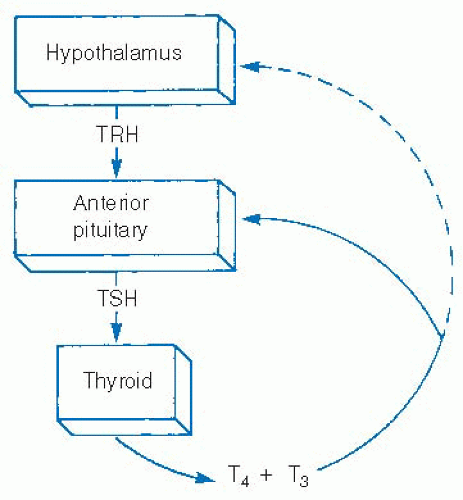
Iodine and thyroid-stimulating hormone (TSH) are the major influences on thyroid hormone secretion and thyroid stimulation. TSH is excreted from the anterior pituitary and is in turn regulated by thyrotropin-releasing hormone (TRH) from the hypothalamus. Both TSH and thyrotropin-releasing hormone excretion are influenced by feedback from serum levels of thyroid hormones (Fig. 28-8). TSH has been shown to increase thyroid hormone production through multiple mechanisms, from increasing iodine uptake by the sodium-iodine symporter to expediting Tg reuptake by thyrocytes as well as multiple steps in thyroid hormone production.74
TSH also stimulates thyrocyte proliferation. It is this effect that has led to the use of TSH suppression as an adjunct in the treatment of thyroid malignancy. Consistent TSH suppression therapy, interrupted only for short periods of TSH stimulation, in order to perform diagnostic studies or ablative treatment with radioactive iodine has long been the mainstay of papillary and follicular thyroid cancer treatment. TSH stimulation has traditionally required withdrawal of exogenous thyroid hormone for several weeks. More recently, however, this stimulation can be obtained through the use of recombinant human TSH (rhTSH) injections without the need for thyroid hormone withdrawal.
NATURAL HISTORY, PATHOLOGY, PATHOGENESIS, AND PATTERNS OF SPREAD
Papillary thyroid cancer, and to a lesser extent follicular thyroid cancer, generally grows as an intraglandular mass before developing lymphatic and distant disease. For this reason, most patients will be alerted to the possibility of the presence of cancer by the discovery of a thyroid mass. Most thyroid masses are, in fact, benign, so the level of clinical suspicion may impact upon the discovery of a malignancy.
A myriad of pathologic processes might present with a mass in the thyroid. Among the benign thyroid masses are adenomatoid nodules, follicular adenomas, and thyroiditis. Adenomatoid nodules are the most common finding to present as a thyroid mass. These may occur as individual masses, but are commonly seen in multinodular glands. Follicular adenomas are benign, but due to the limitations of cytologic evaluation cannot reliably be distinguished from follicular carcinomas. Patients with autoimmune thyroiditis are at risk of developing thyroid lymphomas.
Malignant thyroid masses include papillary and follicular cancer, medullary and anaplastic cancer, lymphoma, squamous cell carcinoma, metastatic tumors to the thyroid, and other rare malignancies. Most of these tumors arise from follicular epithelium. The exceptions are medullary cancer, which arises from parafollicular cells and lymphomas, which arise from intrathyroidal lymphoid cells.
Papillary cancer is the most common thyroid malignancy and makes up approximately 80% of all thyroid cancers.76,77 Follicular cancer makes up approximately 10% of thyroid cancers and the remaining 10 % of tumors are medullary, anaplastic, and other tumors. The distribution of these cancers changes with age. Papillary cancer is more common in patients younger than 40 years of age,
and follicular cancers more common in older patients. Anaplastic cancers are almost exclusively seen in older patients.
and follicular cancers more common in older patients. Anaplastic cancers are almost exclusively seen in older patients.
Papillary Cancer
Papillary architecture is the histopathologic feature that is characteristic of papillary cancer. However, there are multiple additional features that may be seen. The tumors consist grossly of a firm mass within the thyroid gland. Papillary fronds and areas with laminated calcified structures known as psammoma bodies may be present. Crowded follicular epithelial cells with cleaved nuclei, nuclear clearing, prominent nucleoli, and mitotic figures define this cancer.78 Many of these nuclear features can be identified easily in cytologic specimens, making the diagnosis of this tumor by FNA quite accurate.
Earlier in this chapter, the authors reviewed the frequency of occult thyroid cancer and estimated that a large proportion of occult cancers of the thyroid are currently undiscovered. These occult tumors are papillary cancers and are defined by a diameter of <1 cm. The term papillary microcarcinoma has been used to describe these tumors. Their natural history is statistically inconsequential, given that most will go undiscovered and never cause any clinical disease. However, whether these occult tumors are biologically distinct from clinically apparent papillary cancers is unknown. There is recent retrospective data from Hong Kong that suggests that only clinically apparent microcarcinomas are of clinical importance due to the fact that, when compared with occult microcarcinomas, only the overt tumors demonstrated a risk of extrathyroidal or lymphovascular invasion, nodal metastases, and postoperative recurrence.79
The natural history of clinically apparent thyroid tumors consists of progressive enlargement, intraglandular metastases, involvement of adjacent structures, regional lymphatic disease, and distant metastatic disease. This series of events is not thought to necessarily occur in a stepwise manner; intraglandular metastasis and regional nodal involvement are the most common clinical findings for this tumor type.
A common pathologic feature in papillary thyroid cancers is for multiple foci of tumor to occur within the gland.80 It has not been clear whether these are intraglandular metastases or true multifocal tumors. Previous literature suggested that this was more commonly a feature seen in irradiated glands.81 Others found support for a mechanism of intraglandular metastatic spread.82 However, recent investigations have found genotypic distinctions between multifocal tumors in 40% to 50% of patients with papillary cancer.83,84 These data indicate that a significant portion of multifocal tumors arise independently in a gland at risk as opposed to intraglandular spread of tumor.
Others, however, have shown evidence of clonal origin in >85% of these multifocal tumors.85 The papers showing genotypic differences between tumor foci utilized single gene comparisons whereas the paper demonstrating clonality utilized multiple loci in a loss of heterozygosity investigation. Given that tumor development is a multistep process, with multiple genetic events, additional genetic events may occur after intraglandular dissemination. The prognostic impact of multifocality seems to predict an increased risk of recurrence without an impact on survival.86
Regional lymphatic metastases are the second most common pathologic feature of papillary thyroid cancer. These occur in >35% of adults with papillary cancer87 and an even higher proportion, 40% to 75%, in patients older than 40 years of age at diagnosis.88,89,90 The risk of regional lymph node metastasis has been shown to be higher in patients with multifocal disease and in those with intratumoral lymphatic vessels.91,92 The risk of nodal disease is not confined to those with macroscopic primary tumors as some series of microcarcinomas have shown metastatic nodal disease in approximately 50 % of patients.93
The prognostic impact of nodal disease is unclear. Although there is a high rate of nodal disease in young patients, these patients have an excellent prognosis. The impact of age on prognosis is so dominant that the overall significance of nodal disease has been debated. However, in multivariable analyses and in age-matched case-control studies, a worse prognosis emerges for patients with nodal disease.91,94 This is shown for both cancer recurrence and disease-specific survival.86 The greatest impact of nodal disease on survival may be seen in patients older than 60 years of age, a cutoff significantly older than is reflected in the current staging system (where age 45 is thought to be determinant).95
Between 10% and 20% of papillary thyroid cancers will show evidence of local invasion. When this extends beyond perithyroidal fat, adjacent structures may be invaded. Most commonly, extension to strap muscles anterior to the gland may be seen. The clinical significance of this is minimal as these muscles may be resected without significant postoperative sequelae. Tumor or medial compartment nodes may also invade the recurrent laryngeal nerves, the hypopharynx, the esophagus, the larynx, or the trachea. Invasion may range from microscopic to gross invasion of these structures. Preoperative indications of involvement include voice change, dysphagia, and respiratory compromise.
Distant metastatic disease from papillary thyroid cancer is most commonly seen in the lungs. Other distant sites include bone, brain, and, less commonly, other locations. Distant metastases are shown in 2 % to 3 % of papillary thyroid cancers at diagnosis.96,97 Microcarcinomas have been suggested to have an equivalent likelihood of distant metastasis at the time of diagnosis.98 In follow-up, another 3 % developed lung metastases. The risk is greater when follicular variant papillary cancer is present and even higher for macroinvasive follicular carcinoma.97 The prognostic impact of distant metastatic disease is significant with a worse survival when disease is macroscopic and does not concentrate radioiodine.99,100 Adults with macrometastases have been reported to have a 50% survival at 1 year,101 but this can depend significantly on the number and location of the distant metastases.
Distant metastases from DTC appear to be more common in children than in adults. The prognostic impact, however, is less. Children younger than 21 years of age will present with distant disease in approximately 25% of cases, but 10-year cause-specific survival approaches 100%. The need for ongoing management of these cases is indicated by a progression-free survival rate of 66% at 10 years.102
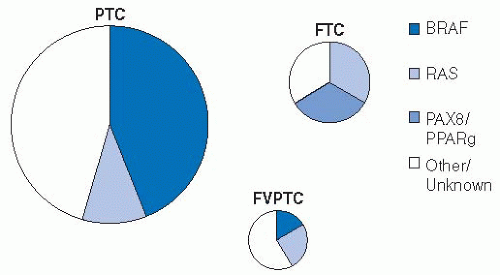
A growing body of evidence shows the high frequency of BRAF mutation in PTC. Next to melanoma, papillary thyroid cancer has the highest frequency of this mutation, ranging from 27% to 81 %.103 The result of these mutations is activation of the mitogen-activated protein kinase (MAPK) pathway leading to tumorogenesis. Other pathways leading to activation of this kinase include ras mutations (more common in follicular thyroid cancers). These pathways to MAPK activation appear to be mutually exclusive mechanisms within individual tumors. There are >40 different BRAF mutations reported, but >90% consist of a T to A transversion at nucleotide 1,799 and lead to an amino acid change at residue 600. This mutation is described as BRAFV600E and causes constitutive activation of the protein, thus driving the MAPK pathway.104
There may be geographic variability in BRAF mutations in PTC with the highest rates shown in Korea (63%-81%).103 The lowest reported rates are in Southern Europe (27%-60%). Interestingly, data from University of California, San Francisco (UCSF) describes an increase in BRAF mutations in papillary cancers treated at that institution over time with a rate of 43 % to 51 % prior to 2001 and a rate of 88 % since that time.105 The prognostic impact of BRAF mutations is under study.104
Follicular Variant of Papillary Thyroid Cancer
The World Health Organization reports 15 variants for papillary thyroid carcinoma.106 The most common of these variants are the follicular, tall cell, and diffuse sclerosing variants (DSVs). In a series of >1,000 cases in 30 years, reviewed with present day diagnostic criteria, classic papillary cancer was seen in 46%, microcarcinoma in 28%, follicular variant in 18%, tall cell in 4%, and diffuse sclerosing in 2 %. Other variants, including insular, oncocytic, and others were seen <1 % of the time.107
While several variants of papillary thyroid cancer exist, the follicular variant of papillary thyroid cancer (FVPTC) is most common. According to Livolsi, this variant was first described in 1960, but was not widely recognized until approximately 20 years later.78 The hallmarks of this tumor are a predominantly follicular pattern with the nuclear features of papillary cancer. The tumors may be encapsulated or unencapsulated, with the encapsulated version difficult to distinguish from follicular neoplasms.78 Although the architecture may resemble a follicular neoplasm, the diagnosis is made on the nuclear features that the tumor presents, most commonly intranuclear inclusions, nuclear grooves, and ground glass nuclei. There is inconsistency seen among pathologists when evaluating thyroid specimens for the presence of FVPTC. In fact, in a series of >85 cases of FVPTC, there was complete agreement by 10 pathologists in <40% of cases.108
Although FVPTC is considered to be the most common subtype of papillary thyroid cancer, recent molecular investigations indicate that this tumor may be distinct from papillary cancer.109 Ras mutations are more common in the FVPTC than in classic papillary cancer and are thought to be associated with the follicular differentiation of these tumors.110 The peroxisome proliferator-activator receptor-γ alteration can also be seen in these tumors and in follicular neoplasms, but not in papillary thyroid cancers of the classic type.109 In addition, BRAF mutations, which are very common in classic papillary cancer, are seen in as few as 12% of FVPTC cases.111 Recent data indicate the FVPTC phenotype may vary with the molecular signature of the tumor.112
The clinical presentation of FVPTC is similar to other thyroid neoplasia. Grossly, the tumor differs from classic papillary thyroid cancer by the presence of a tumor capsule in most cases. The diagnosis is confirmed, however, by nuclear features as opposed to capsular invasion, thereby following a pattern for papillary cancer. Some series indicate that this tumor presents in a manner that is intermediate between papillary and follicular cancers with respect to extrathyroidal extension, lymph node metastases, and distant metastases. Extrathyroidal extension and nodal disease has been shown less commonly in these tumors than in papillary cancers, but still more commonly than in follicular tumors.96 A greater prevalence of multifocality in FVPTC has been shown when compared with papillary cancer.113 Depending on the study, distant disease can be found more commonly in FVPTC than in papillary cancer, but neither tumor has distant metastases as commonly as follicular cancer.96,113 In the authors’ own experience, it was found that although FVPTC tumors are larger at diagnosis, the behavior demonstrates less likelihood for local invasion and a lower recurrence rate.114
Owing to the fact that the diagnosis of FVPTC is made by the nuclear features of the tumor, it is suspected that previous cases have been overlooked. In an effort to evaluate the outcome of these cases, a series of FVPTC cases including those previously classified as follicular adenomas and carcinomas has been performed. This leads to the assessment that most (˜80%) FVPTC are encapsulated tumors, some of which are invasive and some not invasive, but both groups show low incidence of lymph node metastasis and excellent outcomes. The 20% of FVPTC cases that are unencapsulated appear to represent those with a poorer prognosis, although their behavior was shown to be similar to papillary cancer, not worse. Therefore, even within this tumor type there appears to be subtypes that may have distinct behaviors.115 Recent investigation of BRAF mutations in these tumors correlates genotypic findings with histologic appearance and clinical behavior (Fig. 28-9).112
Given that the diagnosis of FVPTC is made by cellular and nuclear features, one might expect that diagnosis of this tumor may be afforded by FNA biopsy. Unfortunately, the sensitivity of cytology is low, estimated as low as 9 % by researchers at the University of Pennsylvania. Intraoperative pathology is better, but still less than optimal at 36%.116 Others have reported more favorable sensitivity with FNA, but still <50%.117
The pathogenesis and presentation of FVPTC may be distinct from classic papillary cancer. However, most reports reflect an overall survival that is similar to classic papillary thyroid cancer.
Other Variants of Papillary Cancer
The third most common variant of papillary cancer, after the classic type and the follicular variant, is the tall cell variant (TCV). This is diagnosed based on the appearance of the follicular cells making up the tumor. Diagnosis is made when >70% of the follicular cells are found to be twice as tall as their basal width. These are often large, locally aggressive tumors and vascular and lymphatic invasion may be seen.78 The diagnostic prevalence of TCV appears to vary between institutions. A review of cases in Sweden found that in a series of papillary cancers, the diagnosis was made in 2 % of cases read by a local pathologist. However, further review by “expert” pathologists led to diagnosis in another 5% of cases.118 The presentation of these tumors is more often in males and in older patients and show increased likelihood of extrathyroidal extension.119 The authors have found that tall cell cancers were more likely to be smaller than 1.5 cm in diameter, but more likely to be extrathyroidal and of higher stage than typical papillary cancers.120 One factor that might impact on this tendency for extrathyroidal extension is a higher rate of expression of c-MET, a tyrosine kinase that influences cellular invasiveness.121
Decreased disease-specific survival has been shown in most series with a mortality as high as 25 %.119,122 The authors’ review of survival was more favorable with a mortality of 5 % although the mean follow-up was <4 years.120 A recent matched-pair analysis comparing tall cell to classic PTC was performed using the
SEER database. Controlling for demographics, extent of disease, and treatment, this found worse disease-specific 5-year survival (82% vs. 91%) in tall cell cancer.123
SEER database. Controlling for demographics, extent of disease, and treatment, this found worse disease-specific 5-year survival (82% vs. 91%) in tall cell cancer.123
The features of the DSV of papillary cancer include diffuse involvement, numerous micropapillary structures with lymphatic permeation, extensive squamous metaplasia, numerous psammoma bodies, marked lymphocytic infiltration, and prominent fibrosis. When compared with classic papillary cancer, the clinical features of these tumors reveal a similar predilection for women, a younger age at the time of diagnosis, a greater incidence of cervical metastases, increased incidence of lung metastases, and decreased disease-free survival on follow-up.124 Others have confirmed that this variant is associated with increased lymph node involvement, increased regional recurrence, increased distant metastases, and decreased survival.125
Tall cell and DSVs of papillary thyroid cancer would appear to have a more aggressive behavior and worse prognosis than classic papillary thyroid cancer. Unfortunately, the surgeon is still limited by incomplete knowledge at the time of surgical treatment of thyroid cancers. This is due to overlapping cytologic features among the various papillary cancer subtypes. While some cytologists have retrospectively determined cytologic features that indicate tall cell cancers, the ability to distinguish these subtypes on biopsy or frozen section in practice is limited.126,127 Surgeons, therefore, are usually required to make decisions about surgical treatment based on clinical features and intraoperative findings.
While subclassification can indicate prognostic differences between various patients with papillary carcinoma, tumor grading may be a more important prognostic factor in multivariable analysis. This may be due to the relative infrequency of each particular papillary variant. However, when taken together, patients presenting with high-grade tumors, represented by nuclear atypia, tumor necrosis, and vascular invasion, do demonstrate decreased survival.128
Follicular Carcinoma
Follicular thyroid cancer represents the second most common thyroid cancer type. Approximately 10% to 15% of thyroid cancers are classified as follicular. These tumors are typically encapsulated, solid neoplasms with minimal colloid, microfollicle formation, and variable degrees of nuclear atypia. This histologic pattern is similar in benign and malignant follicular neoplasms; therefore, the distinction between the adenoma and carcinoma hinges on identification of vascular and/or capsular invasion by tumor.
The behavior of follicular cancer is thought to be somewhat worse than papillary carcinoma. However, this may be secondary to an older age and more advanced stage at diagnosis when compared with papillary cancer.87,129 When controlled for age, gender, and stage, the prognosis of follicular and papillary cancer are similar.129 Mazzaferri and Jhiang report similar 30-year survival and recurrence rates for these tumors types after eliminating those patients found to have distant metastatic disease at diagnosis.87
Tumor stage appears to be critically important in comparing these tumor types, and inherent biologic differences are probably indicated by the 11 % to 18% rate of distant metastatic disease in follicular cancer at the time of diagnosis, more than twice that of papillary cancer.130,131 However, this difference in distant metastatic disease may be partly explained by tumor size at diagnosis. The prevalence of distant disease appears to correlate with tumor size and when tumors of similar size are compared, no difference is seen between follicular and papillary cancer and the likelihood of distant disease.131
The pattern of capsular invasion merits further discussion. Limited invasion into (but not through) the fibrous capsule surrounding the tumor is termed minimally invasive follicular carcinoma. More complete extension through the capsule represents classic follicular carcinoma. The behavior of these two types of follicular cancer differs and some favor an approach to minimally invasive cancer that utilizes conservative surgical treatment only. With regard to vascular invasion, it is associated with a higher rate of distant metastasis.132
Pathologists attempted to define minimally invasive follicular carcinoma as follows: “an encapsulated follicular tumor (not papillary), with only small to medium vessel invasion within or immediately adjacent to the tumor capsule and/or up to full-thickness capsular transgression without accompanying extension into the thyroid parenchyma.”133,134 Unfortunately, there is significant discordance between pathologists as shown in a study from France where diagnostic interpretation was discordant in >50 % of minimally invasive follicular carcinomas when reviewed by a group of experienced pathologists.135 Although the excellent outcomes of patients with minimally invasive carcinoma are encouraging, the authors favor a similar approach to all follicular carcinomas given the limitations of pathology and the potential negative prognostic impact of vascular invasion.
While the pathology of papillary cancer is made in large part on the cellular and nuclear features of the tumor, follicular tumor diagnosis is determined by the tumor architecture, specifically capsular invasion. Cytologically, therefore, papillary cancers are typically diagnosed by FNA biopsy. This is not true for follicular cancers. The cytologic distinction between follicular carcinomas, follicular adenomas, and some cellular adenomatoid nodules is not possible and requires surgical resection to establish the precise tumor pathology. Recent efforts to define the immunohistochemical or genetic distinctions between these various entities hold promise, but are not yet sufficiently sensitive for clinical use.136 Because it is not possible to establish a firm diagnosis of follicular carcinoma preoperatively, it would be helpful to be able to obtain this assessment by intraoperative frozen section. However, capsular invasion may only involve a small portion of the capsule, making the diagnosis of follicular cancer very difficult using standard frozen-section analysis. A diagnostic accuracy of 98 % has been reported when the pathologist is able to process multiple5,6,7,8,9,10,11,12 frozen sections.137 However, this is a significant undertaking and pathologists in the United States have repeatedly emphasized the difficulty in establishing a diagnosis of these cancers on frozen section.138,139 For this reason, most surgeons will try to avoid decision making based on a frozen section in follicular neoplasia.
Invasion of the tumor capsule in follicular carcinoma should be distinguished from extension beyond the thyroid parenchyma. This is an important difference that can confuse the student of thyroid neoplasia. Invasion of the tumor capsule defines the disease of follicular carcinoma but tumor may still remain entirely intrathyroidal. Extension beyond the thyroid parenchyma can occur in a tumor of any histology, and may result in invasion of adjacent structures such as the larynx, trachea, or esophagus. Invasion of these structures demonstrates a biologically aggressive tumor and portends a worse prognosis.140,141,142
The two most common sites of distant disease in follicular cancer are bone and lung. This tumor is more likely than papillary cancer to involve bone, and, not uncommonly, the first manifestation of a follicular cancer will be a bone metastasis. However, because of the greater overall incidence of papillary cancers, that tumor type is more common than follicular in series of metastatic thyroid cancer to bone. In a series from Memorial Sloan-Kettering Cancer Center (MSKCC) with bone metastases from thyroid cancer of various histology, the prognosis was generally poor with survival estimated at 29% after 5 years. Although survival was worst among those with anaplastic and medullary cancer with no 5-year survivors, other histologies revealed a 5-year survival approaching 40%. This rate did not vary between papillary, follicular, or other histologies nor by tumor differentiation.143
Hurthle Cell Carcinoma
Hurthle cell carcinoma is considered to be a variant of follicular carcinoma. These tumors demonstrate large cells with granular cytoplasm and abundant mitochondria. The Hurthle cells themselves are felt to represent a metaplastic response of follicular cells and may be seen in inflammatory as well as neoplastic lesions.78 Although distinguishable from follicular cancer, Hurthle cell carcinoma typically shows a similar histologic pattern and a similar behavior.
The finding of Hurthle cells in cytologic specimens can raise concern for a Hurthle cell neoplasm, but in fact these cells are seen with regularity in benign thyroid nodules. When these cells predominate, then concern for a Hurthle neoplasm is raised. Like other follicular lesions, cytologic diagnosis cannot distinguish malignancy and surgical resection is required. According to experience from the University of Michigan, the likelihood of malignancy in those Hurthle cell lesions undergoing surgical resection is approximately 30%, similar in their experience to that for follicular lesions.144 Other experienced pathologists have indicated a rate of malignancy of 45 % in nodules diagnosed as Hurthle cell lesions by FNA, a higher rate of malignancy than for follicular lesions.145
There has been previous concern that Hurthle cell cancers carry a worse prognosis than follicular cancers. Larger nodules are more likely to be cancer with Hurthle cell cancer in 55% of those over 4 cm versus 13 % in smaller lesions.146 A recent report has used a smaller cut point and integrated age as well. Using these criteria, a lesion >1.5 cm in a patient older than 45 was cancer in 63 % versus 10 % in younger patients with a smaller lesion.147
There has been previous concern that Hurthle cell cancers carry a worse prognosis than follicular cancers. An assessment of SEER data, however, does not show a statistical difference between the two. For a group of >900 patients, 20% were Hurthle cell and 80% were follicular cancer. Ten-year survival was 73 % in Hurthle cell carcinoma and 83 % in follicular cancer. Multivariate analysis revealed that a worse survival was associated with older age (50 years or older), male gender, larger tumor size (>5 cm), nodal metastases, and distant metastases. The histologic type (Hurthle vs. follicular), presence of local extension, extent of thyroidectomy, and radioactive iodine use did not impact the mortality rate.148
Insular Carcinoma
Insular carcinoma is a form of thyroid cancer with aggressive behavior that is intermediate in behavior between the differentiated papillary and follicular cancers and anaplastic carcinoma. An insular pattern may be seen to a variable degree in papillary or follicular cancers, but the prognostic impact of this finding is not significant.149 However, tumors of pure insular pattern are more aggressive than both papillary and follicular cancers. It has been pointed out that the current thyroid cancer staging system distinguishes between differentiated and anaplastic cancer; it does not allow for independent staging of these intermediategrade tumors.150 Insular cancer is associated with nodal disease in 50 % of cases and with distant metastases in an even greater proportion of cases.139 Disease-specific death rates range from 38% to 62% in recent series.151,152
Medullary Carcinoma
Medullary thyroid carcinomas (MTCs) arise from the parafollicular or C cells of the thyroid. These cells, derived from neural crest and part of the diffuse neuroendocrine system, secrete calcitonin and the cancers that derive from these cells also secrete calcitonin. The cancers occur in a sporadic form in approximately 75% of cases. The other 25% occur in a familial form, most commonly associated with an autosomally dominant transmitted syndrome.
Medullary cancers present clinically as firm, encapsulated tumors, often with nodal metastases at presentation. In sporadic cases, the primary tumor is usually unilateral, whereas in familial cases it is typically bilateral. This is explained by the fact that familial susceptibility to medullary cancer leads first to C-cell hyperplasia, and the development of medullary cancer follows this. Multifocal tumors are common in familial medullary cancer.153 The embryologic location of C cells in the upper two-thirds of the thyroid makes this the most common site of MTC.
The pathology of medullary cancers demonstrates a variety of growth patterns. Most often, tumors show a nested, trabecular, or solid growth pattern that invades adjacent thyroid tissue. Cells may be round or spindle shaped.78 Amyloid has long been noted as a common feature in MTC, present in 60% to 80% of tumors.154 Amyloid consists of the fully formed protein of calcitonin hormone.155
Calcitonin can be seen in MTC tumors using immunohistochemical staining. Calcitonin can also be measured in serum, and this is a reliable tumor marker with which to measure the presence or absence of disease. There are few other tissues in the body that secrete calcitonin, and none to any significant amount leaves calcitonin as an accurate serum tumor marker for this disease. Data in the U.S. and Europe suggest that routine screening of serum calcitonin is a cost-effective means of screening for MTC, with a basal serum calcitonin level of >20 pg/mL necessitating further work-up. Recommended preoperative tests for patients with known or suspected MTC include serum calcitonin, calcium, carcinoembryonic antigen (CEA), and RET analysis.156
The familial form of medullary cancer is inherited through autosomal dominant transmission. When it occurs without any other neuroendocrine feature, it is termed familial medullary thyroid cancer (FMTC). When other neuroendocrine tumors are associated, then these tumors form one of the multiple endocrine neoplasia (MEN) type II syndromes. These are listed in Table 28.1. MEN 2A is the most common syndrome and represents >75 % of familial MTC. This syndrome presents with MTC in 90% of patients with a smaller proportion of carriers developing pheochromocytoma (50%) and parathyroid hyperplasia/adenoma formation (20%-30%). The inclusion of FMTC under the heading of MEN 2 is deliberate due to the fact that some kindred of MEN 2 may be incorrectly diagnosed as FMTC due to incomplete history and inadequate follow-up for additional neuroendocrine tumors.157
Familial MTC is associated with a germline mutation of the RET oncogene, located on chromosome 10. This oncogene encodes for a tyrosine kinase that has both intracellular and extracellular components. These germline mutations are usually point mutations in one of the exons 10 to 16 and cause tyrosine kinase activation.157
Extensive work has been done in recent years to characterize the most common mutations in order to predict the clinical behavior of patients with MEN 2.157 This information correlates to the observed clinical behavior of hereditary MTC. It has been observed that MTC, arising as part of MEN 2B, is more aggressive than MTC in MEN 2A or FMTC. MEN 2B is associated with mutations at 918. Cancers with this mutation arise early in life, at a mean age of 3, according to data by Machens et al.158 and may be associated with higher local recurrence and lower survival.156 Mutations associated with MEN 2A (Fig. 28-10) will tend to manifest later in childhood or young adulthood. The most common of these mutations are at codons 618, 620, 634, 790, 791, and 804. The mutations at 768 and 804 appear to be associated with an onset of C-cell hyperplasia and progression to MTC that
occurs at a somewhat later age than many of the other mutations, and MTC with nodal disease is typically not seen before the age of 50.158
occurs at a somewhat later age than many of the other mutations, and MTC with nodal disease is typically not seen before the age of 50.158
TABLE 28.1 Multiple Endocrine Neoplasia 2 (Men 2) and Its Clinical Variants or Syndromes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
Although the age of onset of MTC is variable depending on the specific RET mutation, there may be differences in penetrance related to specific mutation as well. For instance, RET mutation C634Y, causing cysteine to be replaced by tyrosine at that codon, leads to a highly active transformation in the RET gene. This is associated with a high penetrance of MTC (79% by age 30).159 Mutations at this codon are also associated with a 50% penetrance of pheochromocytoma and 10% penetrance of parathyroid hyperplasia.146 In contrast, a mutation such as E768D, which causes glutamate to be replaced by aspartate, has a lower penetrance for MTC (36% at 45 years) and seldom leads to pheochromocytoma or parathyroid hyperplasia.160
Patients presenting as putative sporadic MTC may, in fact, harbor germline mutations. All patients diagnosed with sporadic MTC should be tested due to the fact that previously unrecognized kindreds may emerge or that these patients might harbor spontaneous germline mutations. In a large series of patients thought to be sporadic on clinical grounds, approximately 6% were found to harbor germline mutations.161 Tumor tissue can also be assessed for RET mutations occurring within the thyroid tissue itself. More than 40% of tumors demonstrate these mutations, usually of codons 918 or 634. A worse outcome has been reported for these patients. Of course, only familial cases will have a germline defect in the RET oncogene (e.g., seen in white blood cells) whereas both familial and sporadic disease may harbor a RET oncogene mutation within the thyroid tissue.162
Although the primary tumor in MTC is often well circumscribed and encapsulated, lymph node metastases are common. These nodal metastases tend to first occur in the central compartment, but lateral neck nodes are also common and may require treatment.163 Mediastinal lymph nodes are less accessible for primary surgical sampling, but this area is also frequently involved in more advanced MTC. Data suggest that at the time of thyroidectomy, measurement on intraoperative serum calcium levels 30 minutes after thyroid removal may be a predictor of persistent or distant disease even in patients with no clinical or radiographic evidence of disease.164 However, these data warrant further study. While lymphatic spread is more common, hematogenous spread may occur and result in distant metastases to the lung, liver, and other organs.
When MTC presents clinically, approximately 50% to 80% of the patients will have medial compartment lymph node metastases. A similar proportion will have ipsilateral nodal disease in the jugular nodes. Contralateral nodal metastases will be seen in approximately 20% to 40%.163,165 These high rates of nodal disease have a significant impact on treatment recommendations in MTC and on the ability to normalize serum calcitonin levels after surgical therapy. Detection of lateral neck disease may present a challenge. Ultrasound imaging of the lateral neck has been controversial as up to one-third of scans of the neck may be falsely negative. Some data exist that biochemical marker analysis may be a more sensitive means of determining the need for lateral neck dissection, with serum calcitonin levels >200 pg/mL being suggestive of contralateral neck metastasis and >400 to 500 pg/mL suggestive of distant metastasis.156
In spite of the common feature of nodal disease in MTC and a reduced cure rate when significant nodal disease is present, the natural history of this disease typically manifests slow growth and metastatic spread. Patients with persistent hypercalcitoninemia after definitive treatment may live for many years with no appreciable worsening of their disease. There are exceptions to this rule, typically when more aggressive variants of MTC are seen, and a rapidly fatal course may be seen. These aggressive tumors may be recognized by necrosis, pleomorphism, and multiple mitoses on pathology analysis.
Thyroid Lymphoma
The lymphoid tissues within the thyroid gland can be the tissue of origin for a primary lymphoma of the thyroid. These are unusual, constituting between 1% and 5% of all thyroid tumors. Less than 2% of all extranodal lymphomas will present in the thyroid.166 Most of these will be either mucosa-associated lymphoid tumors, known as MALT lymphomas, or diffuse large B-cell lymphomas. Other lymphoid malignancies present in the thyroid more rarely and include Hodgkin lymphoma, T-cell and other lymphomas, and extramedullary plasmacytomas.
Primary thyroid lymphomas show a female predominance and typically present with painless thyroid enlargement in an elderly female. Approximately 50% will present with aerodigestive tract compressive symptoms.167 Hoarseness is often present and appears to be related to laryngeal edema rather than vocal fold paralysis (Fig. 28-11).168
Preexisting autoimmune disease of the thyroid is commonly seen in thyroid lymphomas according to most series169,170 but more rarely in others.167 The preexistence of autoimmune thyroiditis in these cases not only has pathogenetic implications for lymphoma development, but also creates a dilemma in diagnosis (clinically, cytologically, and pathologically). The development of thyroid lymphoma occurs in most cases with thyroid enlargement over a few months. FNA will demonstrate lymphoid tissue in both Hashimoto thyroiditis and lymphoma, but determining monoclonality of lymphoid cells is critical in order to make the diagnosis of lymphoma. Obtaining adequate material for biopsy may require an open surgical procedure, and depending on the presentation, thyroidectomy may be appropriate, although most frequently chemotherapy without a thyroidectomy represents appropriate management. Jonak et al. have reported good chemotherapy responses with Rituximab plus dose-reduced mitoxantrone, cyclophosphamide, vincristine, and prednisolone.171
 FIGURE 28-11. MRI demonstrating extensive thyroid lymphoma. Note displacement without invasion of the airway. Recurrent laryngeal nerve function was normal. |
There is a female predominance in patients with thyroid lymphoma. Recent efforts to demonstrate monoclonality using polymerase chain reaction methods show promise to improve the diagnostic sensitivity of FNA biopsy for these lymphomas.172
MALT lymphomas are typically indolent, and when diagnosed while still contained within the thyroid parenchyma, stage IE, surgical resection alone may be curative. Diffuse large B-cell lymphoma is more aggressive than MALT lymphoma. It is reported to develop from a transformed MALT lymphoma approximately 40% of the time. At diagnosis, however, disseminated disease is seen in >50% of these cases. These tumors will most often require chemotherapy and radiation with the role of surgery limited to biopsy and airway palliation.173
While this section describes primary lymphoma of the thyroid, it should be recognized that systemic lymphoma may involve the thyroid tissues secondarily. In fact, autopsy series report thyroid gland involvement in 15% of systemic lymphomas174; thus, all patients with lymphoma in the thyroid merit a complete staging evaluation.
Anaplastic Carcinoma
Among the most aggressive of human malignancies, anaplastic thyroid cancer typically presents in the elderly with a rapidly growing neck mass, and compression of the trachea and the esophagus.175 Only 8% of cases present with intrathyroidal disease. Extrathyroidal extension is the rule, making surgical intervention difficult without sacrificing vital structures. Although >40% of patients present with distant metastatic disease, the disease process is so rapid in the locoregional area, that the natural history is one of progressive aerodigestive tract compromise.
It is suspected that frequently anaplastic carcinoma develops from preexisting thyroid disease. In support of this, 80% of cases have a history of a preexisting goiter.176 These cancers may develop from transformation of a DTC.177,178,179 In fact, the vast majority of anaplastic tumors will show some component of papillary, follicular, or Hurthle cell carcinoma, indicating the likely original tumor.168,180 Anaplastic cancer cells are distinct from DTCs in their lack of thyrotropin receptors and in their inability to transport iodine or produce Tg181 and they do not seem to respond to radioactive iodine therapy. Among anaplastic cancers, it has been shown that a low serum thyroxine level is associated with a worse prognosis, but whether this indicates biologic differences between anaplastic tumors or simply reflects total thyroidal tumor burden is not clear.182
In an evaluation of the anaplastic thyroid cancers reported to the National Cancer Institute’s SEER database between 1973 and 2000, 516 cases of anaplastic thyroid cancer were evaluated. Surgical resection was performed in 64% of these cases and radiation therapy was used in 63 %. Patients younger than 60 years, patients with intrathyroidal tumors, and patients treated with surgery followed by radiation were independent predictors of increased survival in multivariate analysis. However, as a whole, the prognosis was poor with 81 % of patients dead of disease at 1 year.183 Even in studies demonstrating improved locoregional control, most patients died of distant metastasis.184,185 There is some evidence that an aggressive treatment plan with surgery, chemotherapy, radiation sensitizers, and IMRT may be of benefit.186,187
Anaplastic tumors typically are composed of varying proportions of spindle, polygonal, and giant cells.188 Papillary cancers may also contain areas with these same cellular features. Although not diagnostic of anaplastic cancer, these papillary cancers are more aggressive and show a lower survival than typical papillary thyroid cancers.189,190 Of more concern, however, is the situation where, within a differentiated tumor, an area shows complete transformation to an anaplastic tumor. These tumors merit aggressive treatment due to a behavior that follows a typical anaplastic pattern. The pathologic diagnosis of even a small focus of poorly differentiated or anaplastic features in a thyroid sample should be considered seriously and treated appropriately. The American Thyroid Association (ATA) is formulating guidelines for the management of anaplastic thyroid cancer that will soon be published.
Metastases to the Thyroid
Microscopic metastases to the thyroid are common when evaluated in autopsy specimens of patients dying with a disseminated cancer of any type. Up to 24 % of these specimens reveal thyroid metastases.191,192 The presentation of clinically apparent metastases in the thyroid is significantly less common than this. However, when a thyroid mass presents in a patient with a known malignancy, metastatic cancer should be considered. FNA should be used to evaluate such a lesion. Typically, diagnosis of such a metastasis will lead to an approach to treatment that is systemic, such as chemotherapy, rather than surgery directed at the thyroid gland. Exceptions may be seen in slow-growing tumors such as renal cell carcinoma that present with a resectable thyroid metastasis,192 where surgical resection can be expected to promptly and effectively reduce the risk of future aerodigestive tract compromise. Renal carcinoma, pulmonary carcinoma, and malignant melanoma seem particularly prone to metastasize to the thyroid gland.
CLINICAL PRESENTATION
The most common presenting sign of thyroid cancer is a thyroid nodule. Although the vast majority of thyroid nodules are benign, all nodules merit careful evaluation for malignancy. Elements of the history and specific clinical symptoms and signs can increase the suspicion of malignancy.
The age and gender of the patient should be considered. Children with nodules present a risk of malignancy that ranges from 15% to 60%.193,194,195 Similarly, nodules in patients older than 60 years of age present an increased risk of malignancy.196,197 The risk of malignancy in a nodule is between two and three times greater in a male than in a female patient.197,198,199
A history of a rapidly enlarging thyroid nodule usually indicates hemorrhage, and this can occur in both benign and malignant disease. These patients usually have thyroid tenderness, discomfort, or pain. Increasing thyroid-related pain should raise concern for malignancy. Hoarseness can be present due to tumor effects on the recurrent laryngeal nerve. This can be seen in benign disease on occasion, but is typically felt to indicate a malignant process. Dysphagia is a common symptom in large multinodular goiters and is gradual at onset in these cases. Dysphagia which increases over several weeks in the setting of a small- or moderate-sized thyroid mass should raise concern for a malignant process involving the inferior constrictor or cervical esophageal musculature. Airway compromise can result from tracheal involvement by tumor or by bilateral recurrent laryngeal nerve paralysis. Both would indicate a likely malignancy.
A personal history of low-dose irradiation is a major consideration when attempting to assess a thyroid nodule. This is associated with an increased risk of both benign and malignant thyroid nodules. The concern for malignancy is significant, and a high degree of suspicion for malignancy should be entertained in these cases.
A family history of thyroid malignancy should be integrated into the assessment of patients with thyroid nodules and trigger the exploration for both papillary thyroid carcinoma and familial medullary carcinoma. For papillary thyroid carcinoma, Cowden syndrome and Gardner syndrome should be considered. Cowden syndrome is associated with hamartomas, breast lesions, gastrointestinal polyps, ovarian cysts, uterine fibroids, and thyroid tumors. Gardner syndrome is associated with colonic polyps and other benign tumors, including osteomas of the facial skeleton. A personal or family history of these related diseases should lead to further evaluation.
The patient suspected of presenting with a familial medullary carcinoma might report chronic diarrhea, which is probably related to the hypercalcitoninemia (or related chemokines). Patients with MEN may report a personal or family history of hypercalcemia, hyperparathyroidism, renal stones, or pheochromocytoma. A history of severe hypertension or symptoms of sweats, flushing, palpitations, and headaches should raise concern for a pheochromocytoma. The fact that, in the past, pheochromocytoma was a major cause of mortality in patients with MEN 2B should be kept in mind while pursuing this history. The possibility of a pheochromocytoma should be excluded in patients with possible or definite MEN 2 before consideration of thyroid or parathyroid surgery.
Examination of the thyroid should focus on the number, size, and laterality of thyroid nodules. Firm nodules are the hallmark of malignant disease. Benign disease tends to be softer on examination, but these physical findings are not considered to be sufficiently accurate to determine management. Benign nodules with hemorrhage may be both firm and tender. Autoimmune thyroiditis may be associated with a diffusely enlarged, rubbery gland, but these glands can harbor DTC and can also give rise to thyroid lymphomas.
Cysts may be evident on examination. Cysts are typically thought to be low risk for malignancy, but cystic cancers do occur. The risk of malignancy in thyroid cysts is 2 % to 9 %.200 However, the ultrasonographic characteristics of the cyst are important. A stepwise rise in malignancy has been shown between
pure cysts (7%), complex cysts (12%), and solid nodules (21 %).201 This relationship emphasizes the importance of ultrasonographic evaluation in thyroid disease.
pure cysts (7%), complex cysts (12%), and solid nodules (21 %).201 This relationship emphasizes the importance of ultrasonographic evaluation in thyroid disease.
The distinction of solitary versus multinodular thyroid disease has been useful in assessing the likelihood of malignancy. As a general rule, solitary nodules are thought to present a greater risk of malignancy than the nodules in a multinodular goiter. However, in a recent, large, retrospective study, the risk of malignancy was found to be approximately 15% for glands with either solitary or multinodular glands when nodules of >10 mm diameter were present. For the individual solitary nodules, the risk of malignancy was greater than for the individual nodules in a multinodular goiter.202 The multinodular glands revealed that the cancer occurred in the largest nodule approximately 70% of the time and that approximately 45 % of the multinodular glands with cancer harbored multifocal cancer.202 The number of nodules may be helpful in predicting malignancy. In patients selected for thyroidectomy at the University of Pennsylvania, the risk of malignancy was approximately 40 % in glands with one or two thyroid nodules, but this risk was reduced by half when more than two nodules were present.203
Assessment of the mobility of the thyroid mass with respect to neck musculature and the laryngotracheal complex is critical and may trigger assessment for local tumor extension by imaging studies. A loss of tissue planes on examination, especially in a rapidly growing mass, should raise concern for anaplastic carcinoma.
Following assessment of the thyroid, the cervical nodes should be evaluated. A delphian node refers to midline adenopathy and may be seen in either thyroid carcinoma or advanced laryngeal cancer. Medial compartment lymph nodes can be difficult to appreciate on examination due to the overlying thyroid tissue and the proximity of the sternoclavicular joints inferiorly. These nodes, however, constitute the primary echelon nodal basin for thyroid cancers. The lateral cervical nodes include those along the jugular vein and in the posterior triangle. The jugular nodes are typically involved before the posterior triangle lymph nodes. High jugular nodes will typically be seen in aerodigestive infections and malignancies. However, thyroid metastases may occur in high jugular nodes, particularly with primary tumors of the upper thyroid pole. These nodes, therefore, should be assessed carefully in patients with thyroid cancer.
Examination of the larynx is critical in thyroid disease. Malignant lesions, and benign lesions less commonly, can result in laryngeal paralysis. Usually, the patient is aware of a recent voice change. Occasionally, however, this paralysis is gradual at onset, and the patient has spontaneously compensated for the paralysis without calling it to the attention of a physician. A preoperative examination on the awake patient is the most sensitive way to discover the paralysis. The authors feel strongly that it is important to document the preoperative laryngeal examination before any thyroid surgery. Preoperative paralysis will increase the concern for malignancy in the assessment of thyroid masses and may also influence intraoperative decisions during thyroid surgery.
The assessment for aerodigestive tract compromise on clinical examination may include indirect or flexible laryngoscopy, and palpation and auscultation of the laryngotracheal complex. If the tumor is compressing or invading the hypopharynx or cervical esophagus, distortion of the hypopharynx may be seen on office laryngoscopy. Pooling of saliva in the hypopharynx may indicate invasion of the pharyngoesophageal junction. Invasion of the laryngotracheal complex may be indicated by immobility of the tumor with respect to these organs, and significant airway compromise may result in stridor, sometimes best appreciated on auscultation of the midline neck.
The remainder of the physical examination in patients under evaluation for thyroid cancer can be directed by symptoms. The lungs are the most common site of involvement by metastatic disease in papillary thyroid cancer, but evidence of this on physical examination is exceedingly rare. New bone pain or discomfort should be fully investigated.
DIAGNOSTIC EVALUATION AND CLINICAL STAGING
When patients present with thyroid nodules following a routine clinical and laboratory examination, the basic workup begins with thyroid function testing and FNA. Ultrasonography is the basic imaging test and provides information of nodule location and architecture as well as an objective measurement of the nodule size. For nodules that are difficult to palpate, ultrasonography is essential in obtaining an accurate FNA. Figure 28-12 describes an algorithm for assessment of thyroid nodules. In the multinodular gland, the largest of the nodules is most likely to reveal cancer, if present in the gland.
Laboratory Testing
When evaluating thyroid nodules, thyroid function testing should include TSH with or without free T4. These tests are almost always normal in patients with thyroid malignancy, so confirmation of normal thyroid function does not reduce the need for cytologic evaluation of the thyroid nodule. One utility of thyroid function tests is in determining whether a patient is presenting with a toxic nodule. These nodules can cause suppression of TSH and typically have a very low rate of malignancy.204,205 The discovery of a suppressed TSH therefore should reduce the clinician’s suspicion of malignancy. In fact, although controversial, some reports suggest that toxic nodules may show cellular abnormalities that can confuse the cytologist; individual circumstances must be considered in the decision whether to perform an FNA on an apparent toxic nodule.
Thyroid antibodies are not routinely obtained in patients with thyroid nodules, but are recommended to a patient suspected of having Hashimoto thyroiditis; these laboratories may support the diagnosis. However, rapid thyroid growth in such a patient may indicate lymphoma, so this should be factored into the assessment.
Tg is normally present in serum at concentrations of approximately 20 to 40 ng/mL. Tg elevation in a previously untreated patient is nonspecific and may be seen in thyroiditis and in hyperthyroidism.206 A markedly elevated Tg may be suggestive of carcinoma, but is not diagnostic.207 This is in distinction to patients followed up for a previous history of differentiated thyroid carcinoma (who have had a thyroidectomy) in whom Tg is a useful tumor marker. When ordered, Tg testing should include simultaneous measurement of anti-Tg antibodies because the presence of antibodies limits the utility of Tg measurement. Tg antibodies commonly interfere in a chemiluminescent Tg assay causing an artifactual lowering of apparent serum Tg levels (frequently being interpreted as undetectable). Radioimmunoassays for serum Tg can report a falsely elevated serum Tg level in the presence of Tg antibodies. In summary, reported serum Tg levels in patients with Tg antibodies are extremely difficult to interpret.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree