Local Presentation
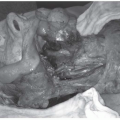
Sarcomas of the head and neck present in multiple ways depending on the numerous potential anatomic sites where disruption of function and contour may result. Local management starts with imaging and physical examination to determine whether soft tissue lesions originate superficial or deep to the investing fascia at the local site and whether bone tumors are intramedullary or have invaded into the extraosseous compartment. Evidence of bone, vascular, or neural invasion (uncommon) should be excluded.
Lesions may originate in the upper aerodigestive tract, paranasal sinus, and skull base with symptoms referable to these areas (e.g., nasal symptoms including obstruction or discharge and ocular proptosis from direct invasion in paranasal sinus tumors; cranial nerve abnormalities in lesions arising in the skull base or masticator space; alteration of voice or airway compromise for laryngeal and hypopharyngeal lesion lesions). Tumors originating in the subcutaneous tissues of the face, neck, or scalp can initially present with a superficial mass.
Both bone and cartilage tumors often present with pain in addition to symptoms of mass and compression. Pain generally relates to infiltration and expansion of bone. In lower-grade lesions, pain can be of long duration and characterized as a prolonged aching sensation, sometimes present for several years. Most often, they present as a slow growing mass or with looseness of dentition. Because of the potential to also arise in the skull base and paranasal sinuses, a wide variety of symptoms are also possible, depending on tumor site. These include cranial neuropathy and visual disturbance (including ophthalmoplegia and proptosis) in addition to pain, the latter being evident in about half the cases.
Metastatic Manifestations
Most sarcomas present with localized disease and metastases are present in only 10% of cases at the time of diagnosis. Generally, the patient is asymptomatic and staging investigations (computed tomography [CT] chest) are needed to demonstrate the metastatic process as the predominant risk is to the lungs. Regional lymph node involvement is unusual but is represented most frequently in certain histologic subtypes, most notably rhabdomyosarcoma, epithelioid, alveolar soft part, clear cell, and synovial sarcomas. Rarely, remote bone marrow metastases are apparent but are seen almost exclusively in rhabdomyosarcoma with associated lymphadenopathy. In osteosarcoma, bone metastases may be present in the absence of lung involvement but this is exceptional.
Evaluation
Initial Assessment. Successful outcome starts with reliance on basic principles and understanding the behavior of the disease and the necessary steps to be undertaken. The overall management of head and neck soft tissue sarcoma is summarized in the treatment algorithm (
Fig. 29-3). Before embarking on treatment, histologic confirmation of the diagnosis is necessary. All soft tissue masses deep to the investing fascia should be considered to be sarcoma until proven otherwise. The same vigilance is needed for bone lesions but here the opinion of an experienced diagnostic radiologist is required. Both bone and soft tissue lesions should be staged with cross-sectional imaging before the biopsy to avoid compromising the planning of appropriate management.
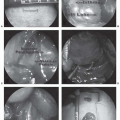
Establishing the Diagnosis. Fine-needle aspiration biopsy (FNAB) is useful for establishing the presence of recurrence (both local and metastatic). However, its use as a primary diagnostic tool is controversial and should only be used where a cytopathologist with extensive experience in sarcoma is available. Core-needle biopsy under local anesthesia provides a more consistent tissue specimen and cell preparation compared with fine-needle aspiration. In our experience, open biopsy of soft tissue sarcoma is rarely indicated but yields a diagnosis when needle biopsy is unsuccessful (about 10% of cases).
Interventional radiologists are often requested to biopsy deepseated lesions using ultrasound or CT guidance. An atraumatic biopsy close to critical structures or where the lesion is difficult to palpate can be accomplished. The radiologist should collaborate with the oncologists who will resect or irradiate the lesion. Needle tracts are potential sites of tumor contamination and the surgeon and radiation oncologist should be involved in planning the optimal biopsy route and be aware of potential contamination.
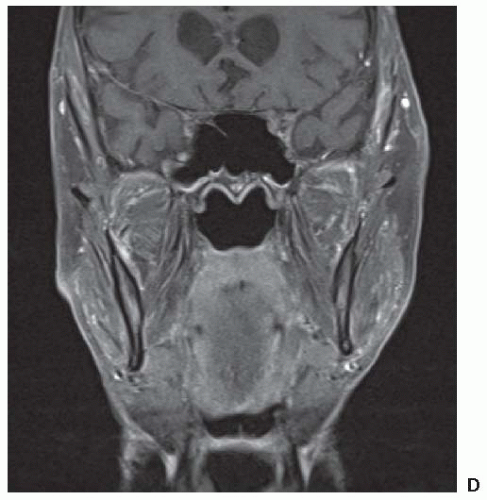
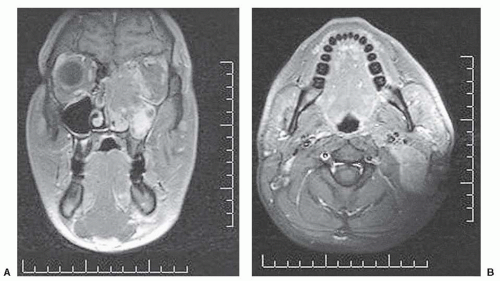
Imaging Local Disease. Both CT scan and magnetic resonance imaging (MRI) are used widely. CT provides better bony detail than MRI, but MRI provides multiplanar images without losing specificity and is likely the best instrument for tumor definition with optimal tissue contrast. Therefore, in those situation where it is important to visualize the matrix of a lesion, CT is preferred. This usually occurs in flat bones including the calvarium. Plain radiography is also useful for bony lesions but has largely been replaced by CT. Both have the advantage of exhibiting calcification in tumor which may be characteristic of certain histologies (e.g., chondrosarcoma and synovial sarcoma). Malignant bone lesions will frequently present with marked permeative bone destruction, illdefined transition to normal bone, aggressive periosteal reaction,
and an accompanying soft tissue mass. In the end, it is our impression that MRI generally provides superior depiction of intraand extraosseous tumor in most malignant tumors of bone. Axial imaging complemented by either coronal or sagittal imaging planes using T1- and T2-weighted SPIN echo sequences most often provides accurate depiction of intra- and extraosseous tumor. To improve conspicuity, these sequences could be augmented by fat-suppressed pulse sequences. The maximum dimension of the tumor must be measured prior to any treatment.
In soft tissue sarcoma, low signal intensity on T1-weighted images and a high signal on T2-weighted images are the usual appearances and can be readily separated from normal soft tissue structures. Again lesion size should be recorded.
Although the diagnostic evaluation may be equally served by CT or MRI, the treatment-planning requirements (e.g., for both surgery and radiotherapy) frequently require additional information provided by the multiplanar capability of MRI and provides additional advantages through MRI/CT image-fusion techniques for radiotherapy treatment planning (
Fig. 29-4).
Radionuclide bone scanning is a sensitive indicator of osteoblastic activity but lacks specificity. It can demonstrate multiple lesions but may also overestimate the extent of intramedullary infiltration when compared with MRI scanning.
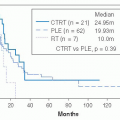
Positron emission tomography (PET) uses radiotracers specific to biologic processes to produce images of regional tissue metabolism with fluorodeoxyglucose, the most commonly employed radiotracer for this purpose. However, the contribution of PET imaging to routine management is currently uncertain. New tracers are being evaluated that may refine the differing roles for PET imaging that include evaluation of (a) the grade and extent of local disease including the potential for “skip” lesions in adjacent bone and (b) the response to initial (i.e., neoadjuvant) treatment as a surrogate to prognosticate on future outcome. It does seem that high- versus low-grade lesions may be distinguished and may even provide an imaging technique for biopsy guidance; in addition, response prediction to induction treatments seems to be possible.
27,28,29,30Evaluation of Metastasis. For metastatic staging, CT of the chest is required, excepting very low-grade lesions where a plain chest radiograph is sufficient. If the underlying diagnosis is rhabdomyosarcoma, a bone scan and bone marrow biopsy is also necessary. Angiography is only rarely required in the evaluation of sarcomas. PET scan is also not widely used but may have applicability in identifying unusual sites of metastases in high-risk subsets (e.g., recurrent high-grade tumors).
31 Technetium scintigraphy is the examination of choice for evaluating the entire skeleton to determine whether there are multiple lesions and is particularly important for diseases with high propensity for bone metastasis such as Ewing sarcoma. Occasionally, intrapulmonary osteoblastic metastases may be demonstrated.
Staging Classifications
Both soft tissue and bone sarcomas are classified according to the TNM stage classification (7th edition).
21 The classification has provided the possibility of combining a number of relevant factors including those addressing behavior (by means of inclusion
of grade) and anatomic factors.
32 However, a major limitation of the staging system is that it does not take into account the anatomic and histologic heterogeneity of these lesions. The system is also optimally designed to stage extremity tumors and is also applicable to the head and neck although lacks subtlety since the T-category size criterion dwarfs the anatomic sites of origin in the head and neck, which tend to be much smaller. An additional issue concerns rhabdomyosarcoma, where two separate descriptions of disease extent exist.
Soft Tissue Sarcoma
“Ordinary” Soft Tissue Sarcoma. The relative rarity of STS, the anatomic heterogeneity of these lesions, and the presence of >30 recognized histologic subtypes of variable grade have made it difficult to establish a functional system that can accurately stage all forms of this disease. The American Joint Committee on Cancer (AJCC) TNM (7th edition) staging system
21 is the most widely employed staging system for STS and is unusual within the overall TNM classification system in that, like the bone classification, it incorporates histologic grade with anatomical disease characteristics. The soft tissue classification employs a three-tiered grading system based largely on the wish to incorporate the French system.
21 In general, the classification is based on an ascending hierarchy of risk depending on whether a lesion is deemed to have no adverse features for relapse or one, two, or three factors present represented by size partitioned at the 5-cm break point, grade (three-tiered system), and lesion depth as “a” (superficial tumor arising outside the investing fascia) or “b” (a deep tumor that arises beneath the fascia or invades the fascia) (
Table 29.4).
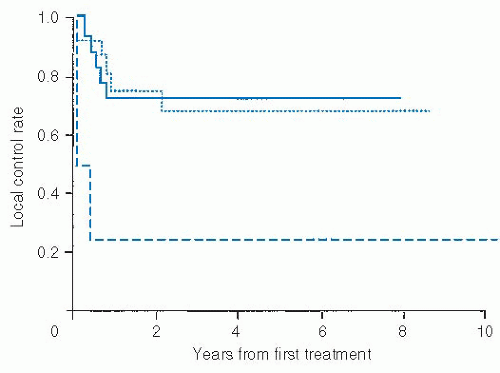
Rhabdomyosarcoma. For nonpleomorphic rhabdomyosarcoma (RMS), the description of disease extent has traditionally been by a postoperative surgical classification developed by the North American Intergroup Rhabdomyosarcoma Study Group (IRSG) more than two decades ago (
Table 29.5). This is not always relevant in the head and neck as such lesions will frequently be treated with aggressive chemoradiotherapy protocols (
Fig. 29-5). Indeed, most lesions would be unresectable and thus classified as group III at diagnosis. They are most often parameningeal in location, considered an “unfavorable” site by the IRSG system, and thus labeled as stage 3. The International Society of Pediatric Oncology (SIOP) has suggested a TNM presurgical staging system more in keeping with contemporary TNM staging for soft tissue sarcoma including a T-category breakpoint at 5 cm (
Table 29.6).
33 This process of staging prior to treatment is also being introduced in North America.
22 Regardless
of the classification used, adequate imaging of regional lymph nodes is imperative in patients in RMS.
Bone Sarcoma. The AJCC TNM classification of bone tumors (
Table 29.7) considers maximum lesion size (with a break point at 8 cm) and the presence discontinuous tumors in the same bone without other distant metastasis. Of interest, metastasis to nonpulmonary sits are distinguished from M1 disease based on lung involvement alone. The staging system is applicable to all primary malignant tumors of bone except multiple myeloma, malignant lymphoma (both having different natural history), and juxtacortical osteosarcoma and chondrosarcoma (both with much more favorable prognosis).
21