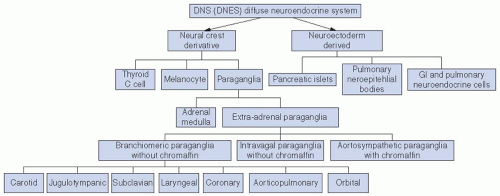
the paraganglia. The carotid body is a discrete oval structure situated behind the carotid bifurcation and receives blood supply directly from the carotid bifurcation via the glomic arteries. The afferent reflex is mediated by a branch of the glossopharyngeal nerve (nerve of Hering) (Fig. 27-2).7
patients with a paraganglioma. Since multicentric tumors may be metachronous, routine follow-up MRI, 111indium pentetreotide (OctreoscanR) or (18)F-DOPA positron emission tomography imaging is indicated.
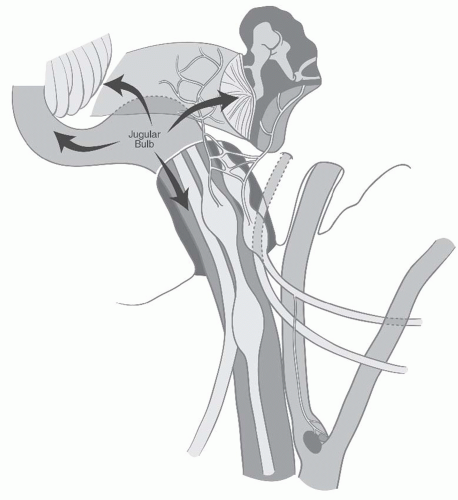
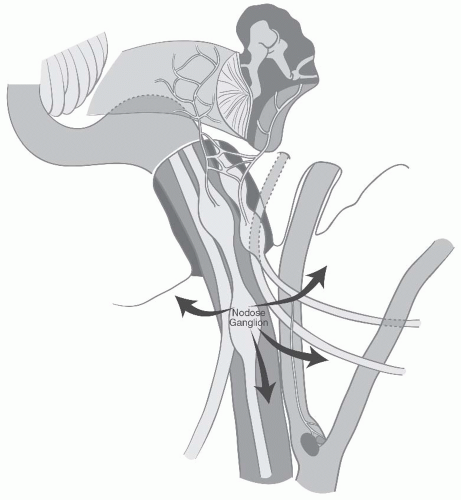
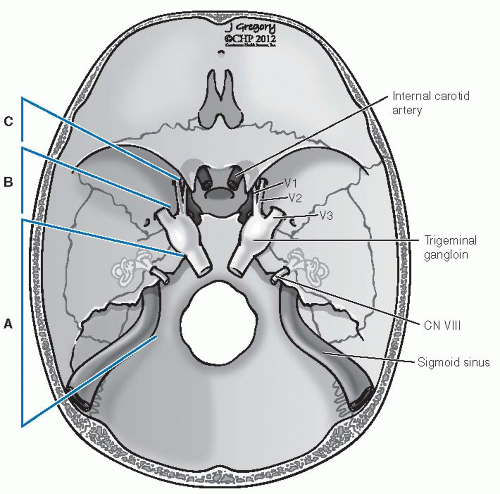
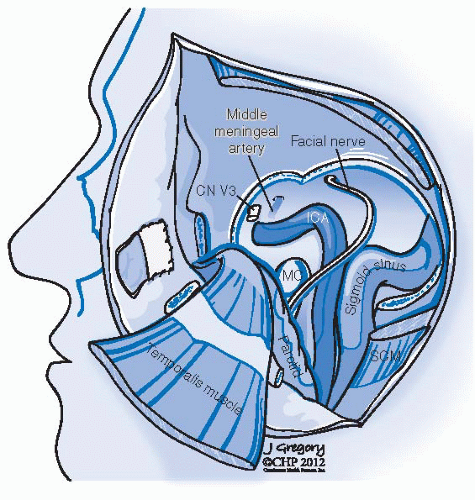
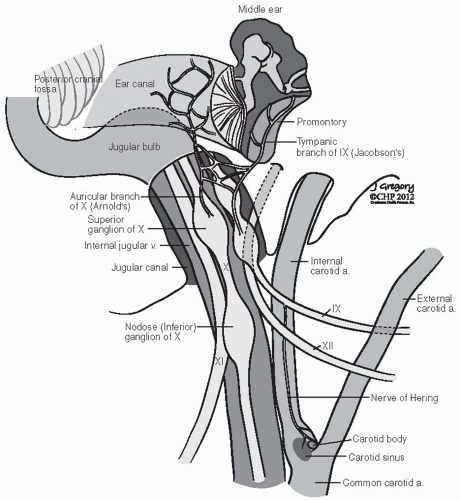 FIGURE 27-2. Schematic representation of lower cranial nerves at the level of jugular foramen. (See color insert.) |
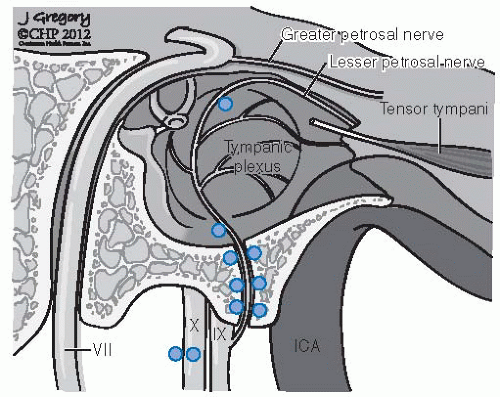 FIGURE 27-3. Distribution of frequent locations for glomus bodies in the temporal bone. (See color insert.) |
predisposition to head and neck paragangliomas and adrenal/extra-adrenal pheochromocytomas.41 This inherited tumorigenic predisposition is transmitted in an autosomal dominant fashion with age-dependent and incomplete penetrance. However, for SDHD located on chromosome 11q, a parent-of-origin effect is revealed as the disease is manifest almost exclusively when the mutation is transmitted from the father. A maternal imprinting has therefore been postulated, but despite the pattern of inheritance, SDHD shows bi-allelic expression in normal tissues and neural crest-derived tissues.20,31
Patients with paraganglioma develop tumors at a younger age than sporadic cases
In PGL1, PGL2, and PGL3, the genetic transmission is autosomal dominant, with highly variable expressivity and reduced penetrance. Genomic imprinting is seen in PGL1: the
paternally transmitted genes lead to tumor development and the maternally transmitted gene gives carrier status without developing tumors
PGL1 and PGL4 show multicentricity and pheochromocytomas
PGL1 has a high degree of penetrance whereas PGL4 shows moderate penetrance
PGL4 shows a marked increase in malignant paragangliomas
PGL3 present exclusively as benign paragangliomas with no multifocality and has no association with pheochromocytomas
Other tumor syndromes: Neurofibromatosis type 1, MEN type 2, and Von Hippel-Lindau predispose to paragangliomas
TABLE 27.1 Genetics of Paraganglioma: SDH- and SDH-related Genes | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
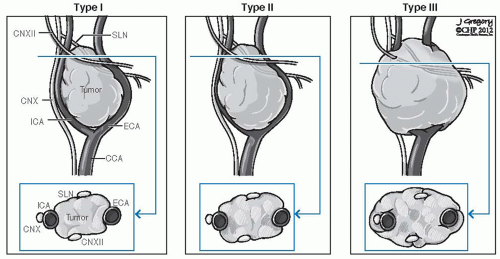 FIGURE 27-6. Shamblin’s classification of carotid body tumors. CCA, common carotid artery; CN, cranial nerve; ECA, external carotid artery; ICA, internal carotid artery; SLN, sentinel lymph node. |
TABLE 27.2 Shamblin’s Surgical Classification of Carotid Body Tumors | ||||||
|---|---|---|---|---|---|---|
|
Growth through Jacobson nerve canal and the hypotympanic air cells leads to involvement of the middle ear and mastoid bone leading to conductive hearing loss, tinnitus, and facial paralysis. Intracranial involvement through the jugular foramen is frequent. Erosion of the jugulocarotid spine leads to involvement of the vertical petrous carotid artery, and further anterior extension leads to involvement of its horizontal portion. More medial extension to the infralabyrinthine air cells lead to involvement of the petrous apex. Further medial extension along this pathway leads to involvement of the clivus and cavernous sinus. Occasionally, jugular paragangliomas will escape the confines of the temporal bone and involve the infratemporal fossa and parapharyngeal space. Lower cranial nerve involvement is frequent and ranges from 38% to 58%.58,59 Multiple cranial nerves are frequently involved in Vernet syndrome (paralysis of CNs IX, X, and XI) or Collet-Sicard syndrome (paralysis of CNs IX, X, XI, and XII). In at least 10% of jugular paragangliomas, one of these sequences is present (Fig. 27-7). The attendant symptomatology is hoarseness, swallowing difficulty, hemipalatal dysfunction with nasal air escape, shoulder motion restriction, and dysarthria due to tongue hemiparalysis. Two surgical classification systems are in wide use today. Both can be used preoperatively based on radiographic findings. The Fisch classification system makes no distinction between tympanic and jugular paragangliomas and is predicated on detailed patterns of progressive disease extension, whereas the Glasscock-Jackson system treats tympanic and jugular paragangliomas differently27,58 (Tables 27.3 and 27.4).
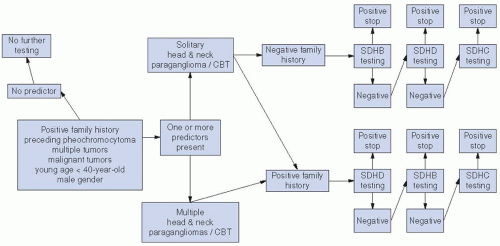
transformation. Attention should be focused on a familial history of paragangliomas, MEN type 2, and Von Hippel-Linday syndromes. Genetic testing for PGL gene should be done if there is a positive family history, and other members of the family should be screened for paragangliomas.
TABLE 27.3 Fisch Classification of Jugulotympanic Paragangliomas | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
TABLE 27.4 Glasscock-Jackson Classification of Jugulotympanic Paragangliomas | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
 FIGURE 27-9. Computed tomography angiogram of a patient with a large paraganglioma. A: Volumetric surface rendering shows anterolateral displacement of the internal carotid artery and narrowing of its lumen. Note the filling of the internal jugular vein with contrast posterior to the common carotid artery, demonstrating the high flow within this tumor. B: Coronal reformat demonstrates involvement of the jugular foramen as well as the hypoglossal canal by tumor. C: Sagittal reformat demonstrates circumferential involvement of the internal carotid artery with anterior displacement, with extensive involvement of the jugular foramen to its intracranial portion. (See color insert for part A.) |
that involve the ICA because of its vulnerability to injury during surgery, or where a planned internal carotid resection is contemplated. An angiographic balloon occlusion test involves the use of a femoral-artery-introduced catheter guided ultimately into the internal carotid, which is temporarily occluded usually at the carotid siphon within the cavernous sinus. This helps to determine whether there will be neurologic deficit.17,19 During occlusion, several methods of neurologic monitoring testing can be utilized. In order of decreasing sensitivity, they are clinical neurologic examination, electroencephalography (EEG), and quantitative blood-flow examination through xenon CT concurrent examination. Xenon CT angiography is a precise quantitative study with the best sensitivity, but is rarely available and cumbersome (Fig. 27-12).68,69
medical comorbidities, the type of paraganglioma in question (vagal, tympanic, jugular, carotid body), the presence of concurrent paragangliomas, the extent of the tumor, as well as any preoperative cranial nerve deficits, whether partial or complete. When surgery is contemplated, the availability of a surgical team with experience in lateral skull base methods, as well as an experienced interventional neuroradiology team, is sought.
is the escape of embolizing particles into the cerebral circulation with all risk of stroke. This can occur through external-internal carotid circulation anastomoses or flow reversal from the arterial supply of the tumor. The advantages of embolization are tumor shrinkage and decreased blood flow that in large tumors have profound benefits. Less intraoperative bleeding is generally associated with a much easier dissection—more obvious tissue planes and less risk to normal anatomic structures, especially cranial nerves. Importantly, such a circumstance lessens the likelihood of requiring transfusion. Larger paragangliomas have multiple arterial feeding vessels that require superselective angiography. Using these methods, each successive embolization devascularizes additional compartments of the tumor until an absence of a tumor “blush” is achieved. It is essential that surgery be performed within 48 hours of embolization to avoid recruitment of collateral circulation. The inflammatory tumor reaction that has been reported following embolization is countered somewhat by the administration of steroids (Fig. 27-15).72,76
tumors usually show marked hyperemia of the vasa nervosum of the sheath. In larger tumors, these nerves—vagus, hypoglossal, and glossopharyngeal—may be intimately involved and their dissection can cause dysfunction.
caution since the cranial nerve rootlets of the glossopharyngeal and the vagus are at their most vulnerable. In jugulotympanic paragangliomas, these rootlets are displaced medially and are thus in a favorable position. If the tumor does not extend into the pars nervosa/medial jugular bulb compartment, preservation of the cranial nerves is desirable.78
nerve deficits are to be expected as these tumors extend medial to the cranial nerve rootlets, within the pars nervosa of the jugular foramen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree