General Principles and Management
Esther X. Vivas
Andrew A. McCall
Paul A. Gardner
Carl H. Snyderman
INTRODUCTION, NATURAL HISTORY, PATHOLOGY, AND PATHOGENESIS
Lateral skull base malignancy is rare. The lateral skull base is an ambiguous term that denotes the area lateral to the central skull base and is generally congruous with the middle cranial fossa and its adjacent areas. It includes the infratemporal skull base and extends from the lateral cavernous sinus to the temporal bone. It encompasses the temporal bone, petrous segment of the internal carotid artery (ICA), the Eustachian tube, and branches of the trigeminal nerve. The lateral skull base can be approached from multiple directions, each approach requiring a different understanding of complex anatomical relationships.
Carcinoma of the temporal bone, including the external auditory canal (EAC), middle ear, and mastoid, is thought to affect approximately one to six people per million in the United States.1,2,3 The true incidence of disease, however, is unknown and varies depending on the publication cited. This problem is compounded by absence of a centralized data-collecting system for temporal bone carcinoma, which is available for other malignancies such as through the Surveillance Epidemiology and Surveillance Results (SEER) database. The SEER database, maintained by the National Cancer Institute, collects information on incidence, prevalence, and survival from specific geographic areas of the US population and reports cancer mortality. Unfortunately, it does not support a category for lateral skull base or temporal bone carcinoma. In addition, there is no standardized staging system for temporal bone cancer. Neither the American Joint Committee on Cancer (AJCC) nor the International Union Against Cancer (IUAC) have published staging guidelines. Currently, the modified University of Pittsburgh staging system (2000) is the most accepted and validated system in use.3,4,5,6
To date, there is no large prospective study on the treatment and outcomes of lateral skull base malignancies. The bulk of peer-reviewed literature consists of retrospective analyses. As a result, information on incidence and prevalence is dominated by reports of independent institutional experiences. Due to these limitations and the inherent rarity of the disease, some larger studies have reported their experience using pooled data from various tumor histology subtypes.5
Overall, primary cancer of the temporal bone is thought to account for <0.2% of all tumors in the head and neck.7 Squamous cell carcinoma (SCCA) is the most common primary tumor type to affect the temporal bone. In following with the overall incidence of SCCA of the upper aerodigestive tract, temporal bone malignancies affect more men than women. Certain reports, however, have shown an approximate even gender distribution.8 Caucasians are much more affected than are Blacks and Hispanics. Primary temporal bone cancer typically arises during the fifth and sixth decades of life.
Primary neoplasms of the lateral skull base include cancers that arise from the bone and cartilaginous tissues of the skull base. The lateral skull base is secondarily involved by neoplasms that arise in skin appendages, the parotid glands, the paranasal sinuses, and the nasopharynx and spread along natural corridor tissue planes. SCCA is by far the most common malignancy to affect the temporal bone, though basal cell carcinoma, adenocarcinoma, adenoid cystic carcinoma, and melanoma have all been reported as possible tumor types in adults.5 Etiology of the disease is not entirely known. For SCCA, correlations with exposure to ultraviolet radiation, frostbite, prior radiation therapy, and even chronic infection have been reported in the literature.3 Aside from SCCA, reports vary on the next most frequent tumor type, with adenoid cystic carcinoma, adenocarcinoma, or basal cell carcinoma coming in as second depending on the particular study.3,9,10,11 In the pediatric population, rhabdomyosarcoma is a rare, but aggressive malignancy that can be encountered in the temporal bone. Basal cell carcinoma is the most common cancer of the pinna and as such is often misleadingly included in reports of primary lateral skull base malignancies. Much more common than primary malignancies of the temporal bone are secondary malignancies from locoregional spread. Upper aerodigestive tract,
parotid gland, and cutaneous cancers comprise the bulk of locoregional disease.
parotid gland, and cutaneous cancers comprise the bulk of locoregional disease.
Primary bone and mesenchymal tumors can also affect the lateral skull base. Chordomas are malignant tumors that are thought to arise from embryologic remnants of the notochord. Outside the spine, the clivus is the most common site. These tumors typically present as midline tumors, but can extend laterally to involve the temporal bone and occipital condyles. Chondrosarcomas of the skull base typically arise in the region of the petroclival synchondrosis and have a more parasagittal location than do chordomas. Patterns of growth are similar with concentric destruction of bone and lateral extension to the temporal bone, occipital condyles, and craniocervical junction.
Metastatic disease can involve the temporal bone and lateral skull base. Spread to the temporal bone can occur hematogenously or through meningeal carcinomatosis, which pathognomonically presents with multiple cranial neuropathies. Primary tumor sites that metastasize to the temporal bone can be from the breast, lungs, kidney, stomach, and prostate.12 When metastatic disease involves the temporal bone, widespread hematogenous metastases to other body sites are often present, and prognosis is poor.
RELEVANT ANATOMY AND PATTERNS OF SPREAD
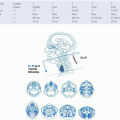
The lateral skull base is a complex anatomic structure that houses important neurovascular structures. The temporal bone articulates with the sphenoid, parietal, occipital, and zygomatic bones. It has four main anatomic components: the squamous, mastoid, petrous, and tympanic parts. It has a pyramidal shape and contributes to the lateral skull base. The squamous part forms the lateral wall of the middle cranial fossa. The mastoid portion houses the mastoid air cell system and vertical segment of the facial nerve to its exit point from the skull base at the stylomastoid foramen. The petrous temporal bone contains the petrous portion of the carotid artery, the otic capsule, petrosal nerves, and the internal auditory canal. The tympanic part houses the bony component of the EAC and at its anterior edge forms the tympanosquamous suture line. The posterior edge of the tympanic ring terminates at the tympanomastoid suture line. The medial two-thirds of the EAC is composed of bone and the lateral third is cartilaginous. The bony-cartilaginous junction is the narrowest segment of the EAC. This junction is an important structure as it serves as a potential pathway for spread of malignant disease. Other potential sites of tumor spread beyond the confines of the temporal bone, including the foramen of Huschke and the fissures of Santorini. The foramen of Huschke refers to a defect in the anterior inferior tympanic ring that usually closes by 5 years of age, but persists in 7% of adults.13 It is a structure that opens into the infratemporal fossa due to incomplete ossification of the anterior bony canal and can allow for tumor extension into the deep lobe of the parotid. The fissures of Santorini are found within the cartilaginous canal and can also allow tumor spread into the parotid gland and periparotid tissues. Nasopharyngeal cancer may spread along the Eustachian tube to gain access to the temporal bone and floor of the middle cranial fossa. Figure 26-1 provides a more comprehensive review of potential avenues for tumor spread. Neoplasms that have a predilection for perineural growth such as adenoid cystic carcinoma and SCCA may track along branches of the facial nerve along its course and extend intratemporally or intracranially. Similarly, perineural invasion by tumor can track along branches of the trigeminal nerve for long distances to reach the middle cranial fossa through Meckel cave or by connections with the facial nerve.
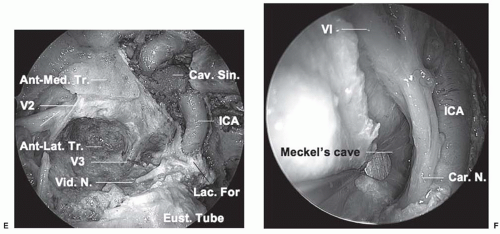
The course of the ICA is a key landmark that defines different surgical approaches to the lateral skull base. The parapharyngeal ICA enters the carotid canal medial to the glenoid
fossa and courses horizontally (in an anteromedial direction) as the petrous segment. The petrous segment of the ICA is situated deep to the Eustachian tube, which runs obliquely across the ICA. Thin bone separates the ICA from the Eustachian tube, measuring about 2 mm at the level of the foramen ovale.14 The lateral pterygoid plate, foramen ovale, foramen spinosum, and spine of the sphenoid are oriented in a line that is superficial to the Eustachian tube. These are useful landmarks for lateral skull base approaches to the petrous ICA. Medially, the petrous segment continues as the paraclival (vertical) segment at foramen lacerum. The pterygoid canal transmitting the Vidian nerve and sometimes artery is a useful landmark for identifying this second genu of the ICA. The Vidian artery is present in a minority of patients, but the Vidian nerve reliably courses superolaterally over the petrous ICA to join the greater superficial petrosal nerve (GSPN) and deep petrosal nerve. Meckel cave and the trigeminal ganglion are bounded by the petrous and paraclival segments of the ICA, the temporal lobe, and the lateral cavernous sinus (Fig. 26-2). The sixth cranial nerve courses superolaterally behind the midportion of the vertical paraclival ICA to Dorello
canal on the superior surface of the temporal bone. It traverses the lateral cavernous sinus just superior to Meckel cave.
fossa and courses horizontally (in an anteromedial direction) as the petrous segment. The petrous segment of the ICA is situated deep to the Eustachian tube, which runs obliquely across the ICA. Thin bone separates the ICA from the Eustachian tube, measuring about 2 mm at the level of the foramen ovale.14 The lateral pterygoid plate, foramen ovale, foramen spinosum, and spine of the sphenoid are oriented in a line that is superficial to the Eustachian tube. These are useful landmarks for lateral skull base approaches to the petrous ICA. Medially, the petrous segment continues as the paraclival (vertical) segment at foramen lacerum. The pterygoid canal transmitting the Vidian nerve and sometimes artery is a useful landmark for identifying this second genu of the ICA. The Vidian artery is present in a minority of patients, but the Vidian nerve reliably courses superolaterally over the petrous ICA to join the greater superficial petrosal nerve (GSPN) and deep petrosal nerve. Meckel cave and the trigeminal ganglion are bounded by the petrous and paraclival segments of the ICA, the temporal lobe, and the lateral cavernous sinus (Fig. 26-2). The sixth cranial nerve courses superolaterally behind the midportion of the vertical paraclival ICA to Dorello
canal on the superior surface of the temporal bone. It traverses the lateral cavernous sinus just superior to Meckel cave.
The cavernous sinus surrounds the sella and encompasses the cavernous segment of the ICA, the nerves to the extraocular muscles, and the first division of the trigeminal nerve. The nerves are all lateral to the ICA and run in the lateral wall of the cavernous sinus before entering the superior orbital fissure. From superior to inferior, the order of the cranial nerves within the lateral wall of the cavernous sinus is III, IV, and V1. The sixth cranial nerve lies medial to the first division of the trigeminal nerve (V1) and runs obliquely through the cavernous sinus lateral to the ICA after exiting Dorello canal. The cavernous sinus can be divided into four compartments based on the course of the ICA: medial, lateral, postero-superior, and antero-inferior. Tumors of the lateral skull base are more likely to involve the lateral (perineural invasion of head and neck malignancy) and antero-inferior (chordomas, chondrosarcomas) compartments.
CLINICAL PRESENTATION
The clinical presentation of incident lateral skull base malignancies can be elusive. It can masquerade as a chronic otitis externa or suppurative otitis media. As a result, the diagnosis of temporal bone malignancies is often delayed until the disease is at an advanced stage. An average 6-month delay in diagnosis has been reported.15 Gidley et al.5 reported a series of 124 patients with SCCA of the temporal bone, of which 71 had incident disease. Within this group, 62 % of the patients presented with otorrhea, followed by otalgia (52%), hearing loss (43%), and facial nerve dysfunction (15.5 %). Additionally, a mass can be frequently seen in the EAC on examination.3 The presence of persistent, deep-seated, unrelenting pain despite adequate treatment for chronic otitis media is an ominous sign and should raise a care provider index of suspicion. This type of pain must be discriminated from other nonmalignant causes; deep otalgia, along with coexistent otorrhea, and abducens nerve palsy is seen with petrous apicitis (Gradenigo syndrome) and deep ear pain that is exacerbated with swallowing or face pain can be seen with Eagle syndrome or geniculate neuralgia, respectively. Spontaneous bloody otorrhea is an unusual presentation of infectious ear disease and as such raises concern for the presence of a more threatening cause. Trismus and pain are ominous signs and indicate tumor extension into the pterygoids and masticator space. Temporal bone carcinoma initially presenting as malignant otitis externa (MOE) has been reported, thus underscoring the importance of early biopsy in suspicious cases.16 Temporal bone involvement by malignancies of salivary gland origin can present with tumors developing insidiously and relatively asymptomatically, with 75 % manifesting with a painless mass and only 6% to 13% manifesting with facial nerve palsy.17 Chondromatous skull base lesions, chordoma, and chondrosarcoma commonly present with headache and diplopia secondary to abducens nerve palsy. Inferolateral extension can result in dysarthria, hoarseness, dysphagia, and aspiration from involvement of the lower cranial nerves.
Metastatic disease to the temporal bone commonly occurs in the setting of more widespread metastatic disease. Temporal bone metastatic lesions can remain clinically silent when tumor is located within the petrous temporal bone because the otic capsule is relatively resistant to neoplastic invasion. If tumor involves the internal auditory canal, or if the otic capsule is breached, symptoms of hearing loss, dizziness, or facial paralysis may ensue.
Perineural extension to the lateral skull base typically involves branches of the trigeminal nerve and may be associated with facial hypesthesia, or pain, or weakness of mastication (Fig. 26-3). If tumor gains access to the lateral cavernous sinus, diplopia secondary to loss of function of extraocular muscles may develop. Most commonly, decreased abduction from a sixth nerve palsy is observed, but ptosis, pupillary dilation, and restriction of gaze in other directions may result from a third nerve palsy.
DIAGNOSTIC EVALUATION AND CLINICAL STAGING
As with any malignancy, a thorough physical examination should be the initial phase in the diagnosis and staging algorithm. A complete head and neck examination is performed with microscopy, assessment of cranial nerve function, and palpation of the parotid gland and cervical lymph nodes. The examiner should also look and test for any temporal lobe or cerebellar signs,
which can be indicative of very advanced disease. New onset seizures likely represent intracranial spread of cancer. A complete audiometric evaluation is required. This provides an objective assessment of the functional status of the middle and inner ear, which is useful during surgical planning and preoperative patient counseling. Conductive losses can be seen with lesions in the external or middle ear, whereas anacusis, tinnitus, and vertigo suggest inner ear involvement.
which can be indicative of very advanced disease. New onset seizures likely represent intracranial spread of cancer. A complete audiometric evaluation is required. This provides an objective assessment of the functional status of the middle and inner ear, which is useful during surgical planning and preoperative patient counseling. Conductive losses can be seen with lesions in the external or middle ear, whereas anacusis, tinnitus, and vertigo suggest inner ear involvement.
Preoperative histologic confirmation of cancer should be obtained whenever possible. However, aside from lesions found in the EAC, access to tissue for biopsy and diagnosis is limited by the anatomic constraints and relative inaccessibility of the lateral skull base. As a result, radiographic imaging has become an integral part of the diagnosis and staging of these malignancies. CT and MRI provide complementary information for most skull base neoplasms. Arriaga et al. determined that preoperative CT can accurately diagnose the pathologic extent of tumor, but not without limitations.18 Some limitations include differentiating fluid and inflamed mucosa from tumor in the middle ear or mastoid, as well as distinguishing inflammation from tumor in the absence of adjacent bony erosion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree