Parotid
The exocrine salivary gland system of the upper aerodigestive tract is composed of two distinct classes of salivary glands. The major salivary glands consist of paired structures in the head and neck region with consistent anatomic drainage patterns within the oral cavity. The largest of these is the parotid gland followed by the submandibular gland and finally the sublingual glands. The minor salivary gland system consists of between 500 to 1,000 small glands located throughout the upper aerodigestive tract. Great density is noted in the region of the lips and the anterior oral cavity but extension of these glands into the oropharynx, tongue, larynx, sinonasal passages as well as the trachea is also demonstrated.
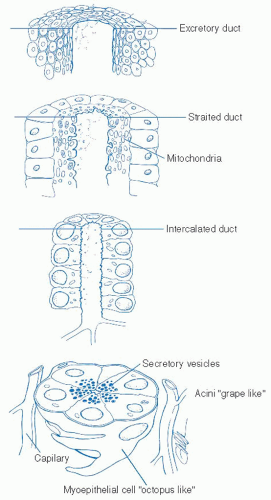
The primary function of the salivary gland system is the production of saliva which is a complex fluid composed of electrolytes, mucopolysaccharides, mucoproteins, immunoglobulins, and lysozymes. The primary digestive enzymes of the salivary gland system are amylase and lipase. The quality of saliva produced by specific glands can be quite variable from the relatively serous saliva produced by the parotid glands to the thicker more mucoid secretion of the minor salivary glands. Saliva plays numerous critical functions within the oral cavity with primary focus on those affecting digestion. Saliva is critical for bolus formation and subsequent lubrication of the bolus to allow the various critically timed stages of deglutition. Enzymatic digestion of food begins with the interaction of the digestive enzymes contained within saliva. Adequate hydration from saliva allows successful speech and articulation. Similarly, saliva plays critical roles in the immune system as well as in the maintenance of dental hygiene. Treatment modalities, whether they be surgical, radiation therapy based, or chemotherapeutic, can have significant impact on salivary production and saliva quality and therefore significantly and negatively impact these critical functions.
The parotid glands are the largest of the major salivary glands weighing between 15 to 30 g each. The parotid gland is a lobulated gland that has its location based in the lateral aspect of the face, anterior and inferior to the auricle. The lateral aspect of the gland is covered by the facial skin. The posterior aspect of the gland rests against the ramus of the mandible. The superior extent of the gland is the zygomatic arch as it continues inferiorly to overlie the posterior belly of the digastric muscle. Laterally, a dense fascia will cover the parotid gland and this fascia will extend all the way from the zygomatic arch inferiorly toward the sternocleidomastoid muscle. Anteriorly, this fascia is contiguous with the fascia overlying the masseter muscle and is referred to as the parotidomasseteric fascia. At the superior and posterior aspect of the gland are several fascial attachments which are critical in the subsequent surgical intervention in and around the gland.
1 Superiorly, thick fibrous attachments connect the gland to the zygoma. The fascia becomes thinner as the gland extends inferiorly in the pretragal region but then becomes quite dense again in the region of the tympanic bone and mastoid. This layer must meticulously be divided to allow access to the stylomastoid foramen and the identification of main trunk of the facial nerve. The most inferior aspect of the parotid gland is referred to as the tail of the parotid and overlies the angle of the ramus, extending down to the sternocleidomastoid muscle. This is the region in which the majority of benign tumors of the parotid gland are located. Final component of the parotid gland extends deeply behind the ramus of the mandible through the stylomandibular tunnel to enter the prestyloid component of the parapharyngeal space.
The primary arterial blood supply of the parotid gland is provided through branches of the extension of the external carotid artery as it continues under the digastric and stylohyoid muscles after giving off the facial artery branch which will course through the submandibular gland. The external carotid artery will then course through the deep parotid tissue to give off the superficial temporal artery. Further continuation of this vessel will give off the internal maxillary artery as it exits the gland. Of note is a
consistent arterial branch, the posterior auricular artery, which will be located approximately 2 mm lateral to the main trunk of the facial nerve and can be a useful landmark during facial nerve identification and subsequent dissection.
The venous system for the gland begins with the superficial temporal and the deep maxillary veins which combine within the gland to form the retromandibular vein. The retromandibular vein will exit the inferior-posterior aspect of the gland and usually be contiguous with the external jugular vein as it drains into the lower neck. If there is no external jugular vein of significance, the retromandibular vein will usually drain into a deep facial vein at the lower aspect of the gland and continue into the internal jugular vein. A critical anatomic relationship provided by the venous anatomy of the gland is that the facial nerve branches will standardly run in a plane just superficial to the retromandibular vein. Therefore, with axial imaging assessment of masses of the parotid gland, the orientation and displacement of the retromandibular vein can give useful information in potential facial nerve branch location.
The primary drainage pathway for the parotid gland is via the parotid duct. This structure is approximately 4 to 7 cm long and exits from the anterior aspect of the gland in the plane along the line from the oral commissure to the lobule of the ear. Upon leaving the gland, the parotid duct will course over the masseter muscle and then make a sharp turn medially as it pierces the buccinator muscle to exit in the oral cavity as the Stensen duct. The Stensen duct is visible as a small papule located in the buccal mucosa opposite the upper second molar. Accessory parotid glands are noted in between 21% and 56 % of patients and will be situated anterior to the gland along the course of the Stensen duct. In most cases, the accessory gland will have ancillary drainage into the parotid duct, but may also have independent drainage.
Critical to the management of any lesion within the parotid gland is a clear understanding of the motor neural supply to the face which is intimately related to the anatomy of the gland. The facial nerve (cranial nerve VII) provides the primary motor neural function to the muscles of facial expression. A thorough understanding of its anatomic course is critical to any surgical management of parotid gland lesions. Originating from the brain stem, the facial nerve has five distinct segments prior to exiting the temporal bone. These include the intracranial pontine segment, the meatal segment through the internal auditory canal, the labyrinthine segment, the tympanic segment, and finally the mastoid segment. The extratemporal component of the facial nerve begins upon its exit from the mastoid bone at the stylomastoid foramen. Familiarity with the intratemporal components of the facial nerve is critical as perineural involvement by tumor may extend proximally requiring resection of a nerve within these regions as well as grafting from the intratemporal nerve to its muscular branches.
The extratemporal facial nerve is directly addressed in most surgeries of the parotid gland, and a clear understanding of its anatomy is critical to successful treatment in this delicate anatomic region. The main trunk of the facial nerve will exit via the stylomastoid foramen which is located laterally and inferiorly to the styloid process at the intersection of the mastoid and the tympanic bones. The nerve will usually give off small branches just upon exiting the stylomastoid foramen to the stylohyoid muscle and the posterior belly of the digastric. At this point, the nerve will then enter the parotid gland and extend for a short course before beginning its branching pattern. The nerve is standardly identified in this region at the main trunk and then dissected in an antegrade fashion to expose its orientation to masses within the gland.
There are numerous critical landmarks which facilitate the location of the main trunk of the facial nerve as it exits the temporal bone (
Table 25.1). The most consistent anatomic landmark is the tympanomastoid suture line. The main trunk of the facial nerve will standardly rest between 2 and 4 mm deep to this.
2,3 The tragal pointer, the apex of the triangle forming the deepest part of the tragal cartilage, is another useful landmark and is defined as the triangular component of the deepest aspect of the tragal cartilage. This will rest approximately 1 cm superior and superficial to the nerve but is a less consistent landmark than the bony landmark of the tympanomastoid suture line.
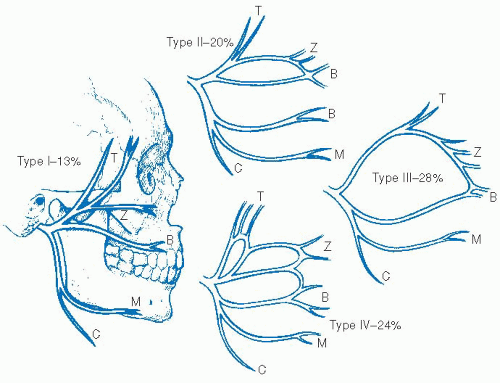
In the dissection of the facial nerve, it is critical that a wide and broad exposure is achieved. This involves freeing the fascial attachments in the pretragal and pretympanic components of the gland as well as broad inferior dissection which allows mobilization of the gland off the digastric muscle. Dissection is then done superiorly toward the digastric ridge where the muscle will enter the mastoid bone, and this similarly gives a good indication of the level at which the facial nerve will rest. With this broad superior and inferior dissection, meticulous dissection can then be done in the region of the tympanomastoid suture line to expose the stylomastoid foramen and the main trunk of the facial nerve. Once in the gland, the main trunk of the facial nerve will demonstrate one of numerous variations in branching pattern to innervate the various muscles of facial expression. The first major split in the nerve is at the pes anserinus. Here the nerve will usually break into two major branches, an upper temporofacial branch and a lower cervicofacial branch. Through various branching patterns described in
Figure 25-1, five major branches will be given off. These include the frontotemporal branch, the orbitozygomatic branch, the buccal branch, the marginal mandibular branch, and the cervical branch.
As noted, clear identification of the facial nerve and its branches is critical to the surgical management of parotid masses, both benign and malignant. If there is an inability to adequately identify the main trunk of the facial nerve and proceed with a standard antegrade dissection, then alternative approaches can be undertaken. A retrograde means of dissection identifies specific branches of the facial nerve distally, once they leave the parotid gland, and then proceeds with dissection in a retrograde fashion as these branches coalesce to form the main branches and the main trunk of the facial nerve. The marginal mandibular branch can be identified inferiorly along the superior and posterior aspect of the submandibular gland and its fascia. The buccal branch will have a consistent location in the midface
as it crosses the masseter in close relation to the parotid duct. Similarly, the orbitozygomatic branch is in a consistent position located on the line between the tragus and the lateral canthus of the orbit. Whichever means of dissection is utilized, be it antegrade or retrograde or some combination, clear identification of the nerve and its anatomy is critical to surgical success.
The sensory supply to the face and auricle surrounding the parotid gland is provided by the great auricular nerve which is formed from the cervical sensory plexus from rootlets C2 and C3. This nerve will course over the sternocleidomastoid muscle and then enter the parotid gland at a point that is usually 8 to 10 mm cephalad to the external jugular vein. The anterior branch of the great auricular nerve will course through the gland and supply the anterior facial skin whereas the posterior branch of the nerve will extend posterior to the lobule to innervate the ear. Preservation of the posterior branch of the great auricular nerve can be achieved with meticulous dissection of this branch through the posterolateral parotid parenchyma and maintenance of its course behind the lobule toward the ear, and studies have demonstrated potential benefit from the preservation of this nerve.
The secretomotor function of the parotid gland is controlled by the parasympathetic autoimmune system via the glossopharyngeal nerve (cranial nerve IX). Preganglionic parasympathetic fiber from the inferior salivary nucleus travel via cranial nerve IX through the jugular foramen where the tympanic branch will then turn back into the skull base via Jacobson nerve to enter the middle ear. These fibers will course along the roof of the middle ear becoming the lesser petrosal nerve which will then exit the skull base at the foramen ovale. The fibers then synapse at the otic ganglion located under the mandibular branch of cranial nerve V3. Postganglionic fibers exit the otic ganglion to join the auricular temporal branch of the trigeminal nerve in the inferotemporal fossa. These parasympathetic fibers supply the innervation for parotid salivary secretory function. Sympathetic innervation is modulated via the superior cervical ganglion sympathetic fibers, which travel along the external carotid system to innervate the gland.
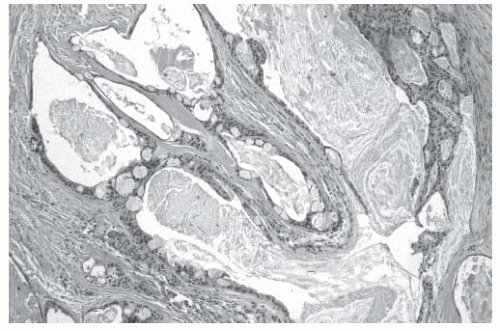
The parotid gland is unique in its lymphatic anatomy. Through embryologic development, lymph nodes in the region of the parotid gland are incorporated into the gland itself and contained within the fascial borders. This is in distinction to the submandibular gland which contains no intraparenchymal lymph nodes. Ninety percent of the lymph nodes within the parotid gland will be located in the superficial lobe. The lymph nodes within the gland provide drainage from the scalp, face, ear, and orbit and primary tumors within these regions can lead to metastases within the parotid gland presenting as parotid masses. Similarly, lymphoma may occur within a lymph node in the parotid gland, presenting as a parotid mass.
Submandibular Gland
The second largest of the major salivary glands are the submandibular glands weighing 7 to 16 g each. These glands are located within the submandibular triangle that is bordered inferiorly by the anterior and posterior belly of the digastric muscle and superiorly by the lower border of the mandible. The majority of the submandibular gland is located below and posterior to the mylohyoid muscle with a smaller component anteriorly and superiorly, which extends deep to the mylohyoid muscle to abut the sublingual glands. This gives the gland a distinct horseshoe shape as it rests within the submandibular triangle. The submandibular gland is surrounded by the middle layer of the deep cervical fascia. This has importance as the marginal mandibular branch of the facial nerve runs in the plane immediately superficial to this fascia along the lateral and superior aspect of the submandibular gland. This becomes an important landmark in submandibular gland surgery designed to preserve the function of the lower branches of the facial nerve. As previously noted, there are no lymph nodes within the gland itself.
4 The majority of lymph nodes associated with the submandibular gland rest superiorly along the facial vasculature as it exits the gland and crosses the mandible. These perifacial nodes deserve a distinct consideration in the setting of cutaneous malignancies as well as oral cavity cancers. Lymph nodes can also rest along the posterior component of the gland where it abuts level IIA.
The arterial supply to the submandibular gland is from the facial branch of the external carotid artery. The facial artery will branch from the ECA and course medially under the digastric and stylohyoid muscle to enter the posterior aspect of the gland. The facial artery will then give off two to four branches to supply the gland and then exit superiorly from the gland over the border of the mandible. Often a small notch can be palpable in the lower border of the mandible where this artery will cross.
The venous supply to the submandibular gland can be quite variable but primarily consist of the anterior facial vein, which travels over the mandible with the artery and then courses on the anterior surface of the gland lateral to its fascia. It then will join the common facial vein and empty into the internal jugular system. On rare occasions, the anterior facial vein may empty into a prominent anterior jugular vein or the external jugular system. The facial vein does have anatomic importance as the marginal mandibular branch of the facial nerve will run in the plane just superior to the nerve and therefore this relationship can be utilized in nerve-preserving maneuvers during submandibular gland surgery. If the vein is located in the lower to mid portion of the gland and then elevated superiorly, this will allow the nerve to be rotated superiorly and provide a protective effect. The posterior venous system of the submandibular gland drains via the posterior facial vein which can be quite prominent. Specific situations that lead to this increased size are if the posterior facial vein provides drainage of the retromandibular vein in the setting of an underdeveloped external jugular system.
The ductal drainage system of the submandibular gland coalesces to form the submandibular duct, which exits the gland and then travels deep to the lingual nerve and medial to the sublingual gland. The duct then enters the floor of the mouth as Wharton duct, which exits at either side of the lingual frenulum in the anterior floor of mouth. The submandibular gland maintains an intimate relationship with the lingual nerve because of its anatomic proximity as well as the attachment of the nerve to the gland via the submandibular ganglion. The lingual nerve enters the submandibular triangle from posteriorly and superiorly as an extension of the mandibular branch of cranial nerve V3. The lingual nerve will then give off a small branch to the mylohyoid muscle which is standardly sacrificed in the majority of submandibular gland surgeries. Parasympathetic secretory fibers from the facial nerve and chorda tympani travel along the lingual nerve to synapse in the submandibular ganglion. From here, the sensory fibers of the lingual nerve will continue anteriorly into the floor of mouth to supply the somatosensory and taste functions of the anterior two-thirds of the tongue. The lingual nerve and submandibular ganglion are readily exposed during surgical exploration with inferior retraction of the gland and superior retraction of the mylohyoid muscle. This allows the gland to be severed from the nerve while maintaining sensory function. This maneuver likewise allows assessment of potential direct neural involvement.
During the same maneuver, the hypoglossal nerve will be evident running in a separate plane deep into the gland. Surrounded by the venae comitantes, the nerve will continue anteromedially to supply motor function to the tongue. The hypoglossal nerve will originate from the skull base exiting at the hypoglossal canal and then travel medially to the internal jugular vein and superficially to the external carotid system as it turns medially to enter the submandibular triangle. The hypoglossal nerve will enter the submandibular triangle inferior and posterior to the junction of the anterior and posterior belly of the digastric muscle at the lesser cornu of the hyoid bone. The submandibular tumors do not routinely directly involve the 12th cranial nerve. Care must still be taken to avoid injury during gland resection with resultant hypoglossal paralysis.
Sublingual and Minor Salivary Glands
The smallest of the major salivary glands are the sublingual glands weighing between 2 and 4 g. The sublingual glands rest in the anterior floor of mouth superior to the mylohyoid muscle. Resting just lateral to the lingual frenulum and covered loosely by the oral cavity mucosa, the sublingual glands have a unique draining system via many small ductules (Rivinus ducts) which exit directly into the floor of mouth. Drainage may also occur via
connections to the submandibular ductal system. The sublingual glands are innervated by the parasympathetic fibers from the seventh cranial nerve.
The minor salivary gland system consists of between 500 and 1,000 variably sized small glands located throughout the upper digestive tract. Ranging in size from 1 to 5 mm, these glands are found throughout the lips, tongue, soft and hard palate, buccal mucosa, pharynx, larynx, upper trachea, nasopharynx, and paranasal sinuses. The majority of the minor salivary glands are concentrated in the region of the lips, tongue, soft and hard palate as well as the buccal mucosa. Postganglionic parasympathetic secretomotor innervation to the minor salivary glands depends on the anatomic region that primarily is via the lingual nerve and branches from the sphenopalatine ganglion.