Chapter 30 General Immune-Based Therapies in the Management of HIV-Infected Patients
The development of highly active antiretroviral drugs, especially protease inhibitors (PIs) or non-nucleoside reverse transcriptase inhibitors (NNRTIs), has resulted in the identification of potent combination regimens that have markedly improved survival and clearly serve as the mainstay of current therapy for HIV-infected patients. However, despite the profound suppression of viral replication that can be seen, the level of immune restoration and the duration of viral suppression associated with such therapies can be limited in some patients.1,2 Further, in many additional patients adverse effects or difficulties in adhering to the rigorous medication regimens decrease the feasibility of long-term therapy with these drugs. Thus identification of effective alternative therapeutic approaches for the treatment of HIV-infected patients is essential.
Given that the vast majority of HIV-related complications, including opportunistic infections and malignancies, are a result of the immunosuppression induced by HIV, rather than being directly mediated by HIV itself, an alternative approach that can complement antiretroviral therapy is to target the immune system directly, in an effort to expand immune function, prevent further immunological deterioration, or improve the host immune response to HIV.3–5
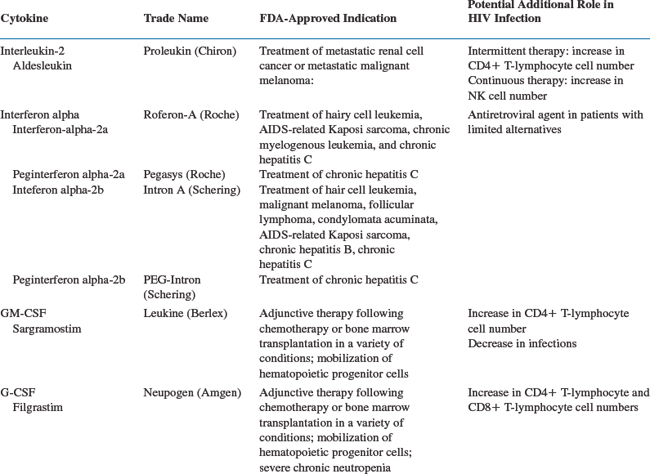
Approaches to immune restoration have included therapy with a variety of cytokines, including IL-2,6 IL-12,7 IL-7, interferon alpha,8,9 interferon gamma,10 and GM-CSF11 (Table 30-1); adoptive immunotherapy using peripheral blood lymphocytes or bone marrow transplants;12–16 passive immunization with hyperimmune globulin;17–19 and T-cell vaccination to reduce anti-CD4+ T-lymphocyte autoimmunity.20 Studies with IL-12 in HIV-infected patients have been limited, and the potential for immunological enhancement has not been well delineated.7 IL-7 is just entering clinical trials. In limited trials conducted in the 1980s, interferon gamma was not associated with benefit, although re-evaluation of its role in the management of certain opportunistic infections may be warranted.10,21 IL-15, which has multiple biological effects, especially related to proliferation and homeostasis of CD8+ T-lymphocyte memory cells, has been proposed as an attractive candidate for immunotherapy but has not yet reached the clinical trial stage. Adoptive immunotherapy studies with small numbers of patients have to date shown no consistent benefit.12–1416 Passive immunization also failed to demonstrate consistent benefit.17–19 Gene therapy approaches utilizing both CD4+ T-lymphocyte and CD8+ T-lymphocyte cells transduced with a variety of constructs that attempt to either improve targeting of HIV-infected cells or to protect cells from HIV infection are also underway but are at a very preliminary stage.15,22,23
IL-2
IL-2 is a cytokine secreted primarily by activated CD4+ T-lymphocyte helper cells.24 It plays a crucial role in the proliferation and differentiation of a variety of cells important to host immune responses, including CD4+ T-lymphocyte helper cells, CD8+ T-lymphocyte cytotoxic cells, antibody producing B cells, natural killer cells, and monocytes/macrophages. Early in vitro studies demonstrated that IL-2 could restore deficient natural killer (NK) cell activity as well as cytomegalovirus (CMV) specific cytotoxicity to cells from patients with AIDS, providing a rationale for clinical trials of IL-2 in this setting.25,26
Early clinical trials of IL-2, which used a variety of doses and durations of treatment, as well as routes of administration, but did not provide long-term therapy, did not demonstrate any long-term immunological or clinical benefit.27–29 Given that IL-2 can activate lymphocytes, which may facilitate replication of HIV, investigators began studies combining IL-2 with antiretroviral therapies.30–32 These studies, which initially focused on patients with relatively intact immune systems, demonstrated that IL-2, when administered intermittently for 5 day cycles approximately every 2 months at a dose of 12–18 million international units (IU)/day by continuous intravenous infusion (CIV), resulted in substantial and sustained increases in CD4+ T-lymphocyte counts, without a concomitant effect on CD8+ T-lymphocyte cells. Based on V-beta analysis, this CD4+ T-lymphocyte cell increase was polyclonal, and was associated with an increase in the number of CD4+ T-lymphocyte cells expressing the high affinity IL-2 receptor (CD25).30 Plasma HIV viral load measured by the branched DNA (bDNA) assay demonstrated a transient increase in some patients that peaked at the end of IL-2 therapy, and returned to baseline within a week of discontinuing IL-2.30–33 Other similar studies noted an increase in NK cell activity, or lymphokine activated killer (LAK) cell activity, during IL-2 therapy.34 Patients with lower CD4+ T-lymphocyte counts (who were being treated exclusively with nucleoside analogues), especially those with CD4+ T-lymphocyte counts less than 100 cells/mm3, did not demonstrate similar CD4+ T-lymphocyte cell responses, but did exhibit more frequent increases in plasma HIV levels that were occasionally sustained.30
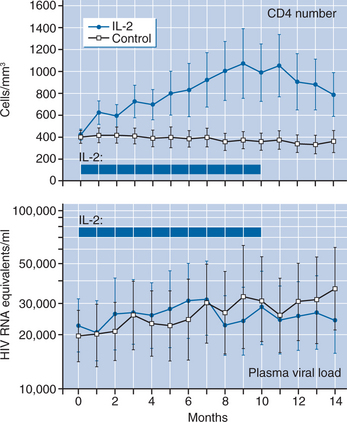
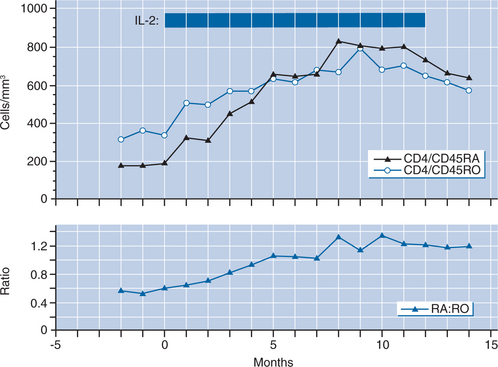
A randomized trial undertaken to evaluate these preliminary observations clearly demonstrated that intermittent IL-2therapy can lead to a substantial and sustained increase in CD4+ T-lymphocyte counts without inducing increases in plasma viral load.6 Sixty patients with CD4+ T-lymphocyte counts greater than 200 cells/mm3 were randomized to receive either licensed antiretroviral drugs combined with intermittent CIV IL-2 or antiretroviral therapy alone. The control group showed a gradual decline in CD4+ T-lymphocyte cell number during the 14 months of the study (−5 cells/month), while the IL-2 group showed a mean increase of 37 cells/month (P < 0.001) (Fig. 30-1), with a concomitant increase in the CD4+ T-lymphocyte percent. There was no difference in the CD8+ T-lymphocyte cell number over time. Additionally, there was a substantial increase in CD4+ T-lymphocyte cells that were CD25 (high-affinity IL-2 receptor) positive, and a preferential expansion of naive CD4+ T-lymphocyte cells (Fig. 30-2). Importantly, no differences in plasma HIV levels were seen between the two groups over time (Fig. 30-1). In an extension phase in which dosing of IL-2 was individualized, the mean CD4+ T-lymphocyte count for the IL-2 group was maintained at approximately double the baseline value through the follow-up period.6 For the control group the mean CD4+ T-lymphocyte count approached that of the IL-2 group during this extension phase.
Two additional randomized trials utilizing intermittent intravenous IL-2 regimens found similar results.35,36 In the first, IL-2 recipients also showed increased delayed type hypersensitivity responses compared to decreases in the control group.35 The second study, which examined the relative efficacy of 3, 4, or 5 day infusions in 81 patients with more severe CD4+ T-lymphocyte depletion (baseline CD4+ T-lymphocyte counts of 100–300 cells/mm3), found a trend toward better responses with longer therapy.36
While CIV IL-2 therapy is associated with substantial immunological effects, the need for continuous infusions, with the associated high cost, inconvenience, and drug toxicities as well as catheter related complications, has led to a focus on alternative routes of administration. Studies using subcutaneous (SC) PEG IL-2 (which is no longer being manufactured) found inadequate immunological enhancement.35–39 However, standard IL-2 administered SC twice daily (usual starting dose, 4.5–7.5 million IU bid) is similar in activity to CIV IL-2, but is more convenient and better tolerated.37,40,41
A number of randomized trials have focused on optimizing the administration of SC IL-2. One study in which patients were randomized to either monthly or every other month cycles, and to two doses (1.5 or 7.5 million IU SC twice a day) of IL-2 found the higher dose induced significantly greater CD4+ T-lymphocyte responses, and that monthly therapy led to more rapid responses.42 The inconvenience of monthly therapy probably does not warrant the more frequent dosing schedule, however. Three studies comparing three doses (1.5, 4.5, and 7.5 million IU twice a day) to antiretrovirals alone found a dose-dependent increase in CD4+ T-lymphocyte cell number, though the two higher doses both induced substantial CD4+ T-lymphocyte count increases.43–45 In all of these randomized trials, no harmful long-term effect of IL-2 on viral load was seen; transient increases in plasma HIV RNA levels can be seen at day 5 in <5% of SC cycles.37
Most studies have found no evidence of enhanced viral suppression in patients receiving IL-2. However, a meta-analysis of long-term follow-up of three CIV studies suggested that IL-2 therapy led to improvements in viral load, and a controlled trial of 82 patients, over 50% of whom were receiving PI therapy, similarly suggested improved viral control in IL-2 recipients.46,47 However, in the largest controlled trial published to date (511 patients), IL-2 therapy did not have a significant impact on viral load after 12 months.48
The development of highly active antiretroviral therapy (HAART) has provided the opportunity to examine the potential role of IL-2 in patients in whom a high level of viral suppression is achieved. ACTG 328 examined the effect of IL-2 in a randomized trial of PI-naive patients with baseline CD4+ T-lymphocyte counts between 50 and 350 cells/mm3, who received 12 weeks of HAART and had suppressed plasma HIV RNA levels to less than 5000 copies/mL.41 Median CD4+ T-lymphocyte counts were significantly higher in the IL-2 recipients (614 cells/mm3 for SC IL-2 and 800 cells/mm3 for initial CIV IL-2 vs 396 cells/mm3 for controls), in the setting of good viral control (∼80% of patients had <50 HIV RNA copies/mL). Similar results were observed in ANRS 079, in which patients randomized to IL-2 plus HAART had a CD4+ T-lymphocyte count increase of 865 cells/mm3, compared to 262 cells/mm3 in patients receiving HAART alone.49
Since a proportion of patients receiving HAART will be immunologic nonresponders (minimal or no increase in CD4+ T-lymphocyte count), the ability of IL-2 to increase CD4+ T-lymphocyte cell number in this setting has also been studied. In three randomized studies of patients with CD4+ T-lymphocyte counts <200–250 cells/mm3 and viral loads <50–1000 copies/mL following 6–9 months of HAART therapy, intermittent SC IL-2 therapy induced a significant increase in CD4+ T-lymphocyte counts within 12–24 weeks.50–52
An alternative approach to intermittent IL-2 therapy has been the continuous administration of lower doses of SC IL-2, either daily using IL-2 or twice weekly using PEG IL-2. Preliminary studies with such continuous therapy demonstrated increases in eosinophils, NK cell number, and NK and LAK cell activity, but most studies have not demonstrated an increase in CD4+ T-lymphocyte or CD8+ T-lymphocyte cell number.38,39,53 A small study (11 patients) observed transient increases in CD4+ T-lymphocyte cell number with continuous three times per week therapy, with tachyphylaxis developing as therapy was continued.54 A randomized study compared the effects of daily SC IL-2 (1.2 million IU m−2 day−1) administered for 6 months to antiretroviral therapy alone in 115 patients (CD41 T-lymphocyte counts <300 cells/mm3).55 Significant increases in NK and eosinophil cell number were seen in the IL-2 group. Although an early (weeks 4 and 8) increase in CD4+ T-lymphocyte cell number was seen, significant differences between the two groups were not sustained. A significant increase in the CD4+ T-lymphocyte percent and the CD4+ T-lymphocyte:CD81 T-lymphocyte ratio was driven in part by a significant decline in the CD8+ T-lymphocytecell number in the IL-2 group.
IL-2 administration is associated with a large number of side effects that are usually dose related, and that tend to be less frequent and less severe with SC therapy.6,35,37,40 The most common dose-limiting toxicities are related to the constitutional symptoms that invariably occur at doses greater than3 million IU/day; fevers, chills, rigors, sweats, muscle and joint pains, nausea and vomiting are all common.6,40 Additional toxicities include fluid retention due to a capillary leak syndrome; elevations in hepatic enzymes and bilirubin; acalculous cholecystitis; diarrhea; renal dysfunction that is due in part to the intravascular volume depletion resulting from the capillary leak, as well as to the use of nonsteroidal antiinflammatory drugs; metabolic abnormalities, including hyponatremia, hypocalcemia, hypomagnesemia, hypoalbuminemia, and phosphate abnormalities; hypothyroidism; cardiomyopathy and congestive heart failure; neurological abnormalities; mucositis; rash, that is occasionally desquamative; and hypotension. While some of these toxicities are potentially life-threatening, such severe toxicities are rarely seen at the doses used in HIV-infected patients. Many side effects that occur can be diminished by administration of adjunctive medications (Table 30-2). Prednisone can blunt some of the toxicities (and TNF-alpha production), but also simultaneously blunts the CD4+ T-lymphocyte cell increase.56 Most of the side effects that develop during IL-2 therapy will improve very quickly (within a few days) of discontinuing IL-2.
Table 30-2 Management of IL-2 Related Toxicities
| Adverse Effect | Management |
|---|---|
| Constitutional symptoms (fever, chills, muscle and joint aches, fatigue) | Ibuprofen (200–600 mg q6 h) alternating with acetaminophen (650 mg q6 h); may be started prior to IL-2 |
| Nausea/vomiting | Prochlorperazine (10 mg PO or 25 mg PR q4–6 h) or ondansetron (0.15 mg/kg IV (10 mg for 70 kg) or 8–16 mg PO q6–8 h) |
| Diarrhea | Loperamide (2 mg q4–6 h PO) or Lomotil (1–2 Tab. qid) |
| Hypotension | Increase oral fluid intake, or administer fluid bolus (e.g., 500 cc normal saline) |
| Mucositis | 2% solution of kaolin pectate, xylocaine, and benadryl (swish prn) |
| Anxiety/insomnia | Lorazepam (1 mg PO q6–8 h prn); flurazepam, diphenhydramine are alternatives; avoid in patients with any mental status changes |
| Rash or itching | Diphenhydramine (25–50 mg PO q6 h prn) or hydroxyzine (25 mg PO tid prn) |
| Injection site reaction | Apply heat prn; rotate sites |
| Creatinine elevation | Fluids as above; discontinue meds that may be contributing, especially nonsteroidals |
| Hyponatremia | Follow closely for values below 130. Normal saline supplementation (50–100 cc/h) for values below 128. Discontinue IL-2 for values below 125 |
| Hypomagnesemia | Oral magnesium supplementation |
| Hypocalcemia | Oral calcium supplementation (may be due to low albumin) |
| Dyspepsia | Ranitidine (150 mg PO q12 h) or cimetidine 300 mg PO q6 h) |
| Other significant toxicities or if above toxicities do not respond to therapy | Discontinue IL-2 |
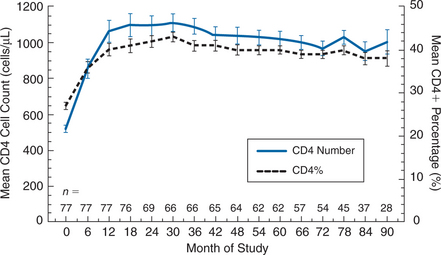
In patients who have demonstrated an immunological benefit from IL-2 therapy, patient inconvenience and discomfort can be decreased by individualizing the dosing regimen.57 A strategy designed to maintain the CD4+ T-lymphocyte count above a threshold level (e.g., double the baseline value, or a count of 800 or 1000 cells/mm3) can often result in decreasing the frequency of an IL-2 cycle to yearly or less often. A recent study of patients who had been treated intermittently with IL-2 in an individualized fashion for a mean of nearly 6 years, during which time a mean of 10 cycles were administered, found that the average recent cycling interval was 39 months.57 This infrequent cycling was sufficient to maintain the median CD4+ T-lymphocyte count at ∼1000 cells/μL (Fig. 30-3).
While substantial CD4+ T-lymphocyte count increases can be induced by intermittent IL-2, the clinical benefit of these immunological changes has not yet been demonstrated. Studies examining the function of CD4+ T-lymphocyte cells in patients receiving IL-2 suggest that the functionality of CD4+ T-lymphocyte cells pre-IL-2 and post-IL-2 appear overall to be similar.58,59 In a substudy of ACTG 328, IL-2treated patients did not have improved immunization responses compared to controls, despite significantly higher CD4+ T-lymphocyte cell counts. The number of substudy participants was small, however, and the two groups were not well balanced in their baseline pre-HAART characteristics.60 In a larger randomized study of patients with higher baseline CD4+ T-lymphocyte cell counts, an improvement in antibody responses to tetatus vaccination and in proliferative responses to p24 was noted in the IL-2 group compared to controls.49 Two studies, one a meta-analysis of three trials, found a trend toward fewer opportunistic infections in patients receivingIL-2, suggesting clinical benefit.41,47 The true clinical efficacy of IL-2 therapy, i.e., the ability of IL-2 to prevent opportunistic infections and death, will need to be addressed in phase III studies with clinical endpoints; two such studies are currently underway, one in patients with >300 CD4+ T-lymphocyte cells/mm3, and the other in patients with <300 CD4+ T-lymphocyte cells/mm3 at baseline.61,62 Evidence of clinical benefit is needed before IL-2 can be broadly recommended for the management of HIV-infected patients.
In addition to its ability to increase CD4+ T-lymphocyte counts, the potential for IL-2 to accelerate clearance of latent reservoirs of HIV has also been studied. While initial studies suggested that it was very difficult to culture HIV in a proportion of patients who had received IL-2 together with HAART therapy,63 studies examining viral rebound following discontinuation of antiretroviral therapy found that in all patients plasma HIV levels increased, with no difference in the kinetics of rebound in IL-2 and non-IL-2 recipients.64 Moreover, in a randomized trial of 56 patients initiating HAART, IL-2 had no effect on frequency of residual viral replication or the amount of proviral DNA.65
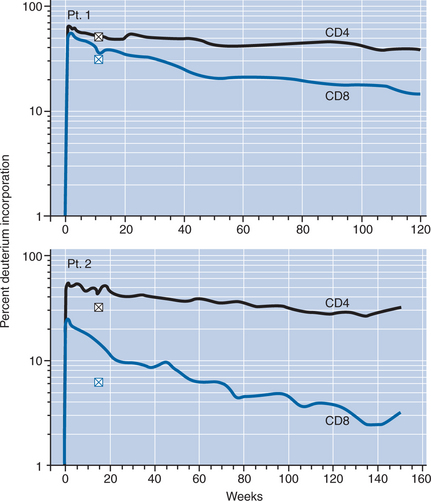
Recent studies have demonstrated that despite significant T cell proliferation during IL-2 administration, the main mechanism of CD4+ T-lymphocyte cell expansion is due to decreased T cell turnover and improved survival of CD4+ T-lymphocyte cells (Fig. 30-4).66,67 The decreased turnover and improved survival affects predominantly CD4+ T-lymphocyte cells of naive and central memory phenotype, which are also the two subsets that are expanded preferentially by IL-2.66–68 Among the expanded cells is a unique, polyclonal naive CD4+ T-lymphocyte cell population characterized by CD25 expression (CD41 CD45RO–CD251) that shares some features with regulatory T cells.68,69
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree