Introduction
Gastrointestinal (GI) toxicities are common side effects of most chemotherapy agents. Their effect on rapidly dividing GI tract cells can lead to mucosal inflammation, ulceration, and perforation. The most common GI toxicities include oral mucositis (see Chapter 5 ), dysphagia, odynophagia, esophagitis, gastritis, nausea and vomiting, enterocolitis, diarrhea, constipation, and hepatotoxicity. Other less common manifestations include GI hemorrhage, bowel perforation, pancreatitis, malabsorption, and infections of the GI tract as a result of chemotherapy-induced immunosuppression. This chapter highlights the mechanisms of chemotherapy-induced GI toxicities and important pharmacological and nonpharmacological management strategies.
Esophageal Complications of Chemotherapy
Clinical esophagitis is observed in 1% to 3% of cancer patients undergoing anticancer treatment and is most commonly due to direct toxicity of chemotherapy or radiation therapy. Patients who are on corticosteroids or are immunosuppressed may develop esophagitis caused by a bacterial, fungal, or viral infection. Candida species, herpes simplex virus (HSV), cytomegalovirus (CMV), and varicella zoster virus (VZV) comprise the majority of pathogens. Gastric acid suppressants (e.g., proton pump inhibitors) can contribute to fungal and bacterial colonization of the upper GI tract, predisposing to infectious esophagitis. Other risk factors include gastroesophageal reflux disease (GERD) and pill-induced esophagitis.
Chemotherapy implicated in esophagitis: A number of chemotherapy agents, including doxorubicin, vinblastine, 5-fluorouracil (5-FU), methotrexate, and dactinomycin, have been associated with esophagitis. However, the incidence of chemotherapy-induced esophagitis is low and is more commonly observed in combination with thoracic radiation therapy. The pathophysiology of esophagitis due to concurrent chemoradiotherapy is complex and multifactorial, but is believed to involve direct cytotoxic injury followed by secondary insult due to the release of proinflammatory cytokines. Agents such as cyclophosphamide, 5-FU, doxorubicin, and dactinomycin also act as radiosensitizers and can exacerbate the cytotoxic effects of radiation.
Esophagitis frequently presents with retrosternal burning, dysphagia, and odynophagia. Subsequent reduced oral intake can lead to dehydration, weakness, need for alternate feeding routes, and treatment interruption. A grading system for esophagitis is described in Table 6.1 .
| Adverse Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|
| Enterocolitis | Asymptomatic; clinical or diagnostic observations only; intervention not indicated | Abdominal pain; mucus or blood in stool | Severe abdominal pain; change in bowel habits; medical intervention indicated; peritoneal signs | Life-threatening consequences; urgent intervention indicated | Death |
| Constipation | Occasional or intermittent symptoms; occasional use of stool softeners, laxatives, dietary modification, or enema | Persistent symptoms with regular use of laxatives or enemas; limiting instrumental ADL | Obstipation with manual evacuation indicated; limiting self-care ADL | Life-threatening consequences; urgent intervention indicated | Death |
| Diarrhea | Increase of <4 stools per day over baseline; mild increase in ostomy output compared with baseline | Increase of 4–6 stools per day over baseline; moderate increase in ostomy output compared with baseline | Increase of ≥7 stools per day over baseline; incontinence; hospitalization indicated; severe increase in ostomy output compared with baseline; limiting self-care ADL | Life-threatening consequences; urgent intervention indicated | Death |
| Esophagitis | Asymptomatic; clinical or diagnostic observations only; intervention not indicated | Symptomatic; altered eating/swallowing; oral supplements indicated | Severely altered eating/swallowing; tube feeding, TPN, or hospitalization indicated | Life-threatening consequences; urgent operative intervention indicated | Death |
| Nausea | Loss of appetite without alteration in eating habits | Oral intake decreased without significant weight loss, dehydration, or malnutrition | Inadequate oral caloric or fluid intake; tube feeding, TPN, or hospitalization indicated | – | – |
| Vomiting | 1–2 episodes (separated by 5 min) in 24 h | 3–5 episodes (separated by 5 min) in 24 h | ≥6 episodes (separated by 5 min) in 24 h; tube feeding, TPN, or hospitalization indicated | Life-threatening consequences; urgent intervention indicated | Death |
Management of esophagitis: Management depends on the degree of clinical impact, etiology, and other patient-related factors. Most cases are treated with conservative measures such as hydration; soft, bland diet; and adequate pain control. Patients should be instructed to avoid hot beverages, spicy foods, citrus, alcohol, and tobacco. Use of antacid therapy such as proton pump inhibitors, topical lidocaine, or topical soothing agents such as “Magic Mouthwash” is also recommended before meals to facilitate swallowing. Sucralfate can be used as an adjunct medication in the treatment of pill-induced esophagitis because it can hasten the healing rate by adhering to esophageal ulcers. More severe cases may require switching to a liquid diet with high-calorie supplements for nutritional support, and analgesics such as narcotics. Hospitalization may be necessary to provide nutrition via the percutaneous or parenteral route in these cases. Endoscopic evaluation is not commonly indicated and can be complicated by perforation. However, endoscopy may be therapeutic in patients with known or suspected esophageal strictures because the surgical dilation of esophageal strictures is usually associated with excellent results, and can be diagnostic in providing etiology of infectious esophagitis.
Evaluation of esophagitis: Workup of a patient with suspected esophagitis should include a good oral examination and consideration for a smear and culture of oral mucosa for Candida . Candidal infection is more common in immunocompromised patients and may or may not be associated with oropharyngeal thrush. The hallmark of esophageal candidiasis is odynophagia, although many patients may be asymptomatic. Treatment involves azole derivatives, such as fluconazole. If definitive diagnosis is not possible or inconclusive, empiric antifungal therapy should be considered due to the risk of esophageal mucosa sloughing and stricture or fistulae formation.
Viral esophagitis: HSV esophagitis usually arises from reactivation of latent virus during treatment-induced immunosuppression. Patients with oral lesions suggestive for HSV infection along with dysphagia or odynophagia are likely to have HSV esophagitis, and culture and histological examination of the oral mucosa are recommended. The treatment of choice is acyclovir. For patients who are able to take oral medications, acyclovir 400 mg PO 5 times a day for 14 to 21 days is effective. For patients who cannot tolerate oral therapy, intravenous acyclovir can be given 5 mg/kg every 8 hours for 7 to 14 days and as soon as their symptoms improve, they can be switched to oral therapy for completion of the therapeutic course. Foscarnet can be used with caution in patients with acyclovir resistance.
Chemotherapy-Induced Nausea and Vomiting
Chemotherapy-induced nausea and vomiting (CINV) remains one of the most distressing and dreaded adverse events associated with chemotherapy. Up to 80% of patients receiving chemotherapy have CINV; however, the incidence and severity depend on the chemotherapy regimen, dosage, duration, and patient risk factors. CINV has been associated with poor adherence to chemotherapy, disruption of treatment schedules, impairment of functional activity, and significant negative impact on quality of life. Uncontrolled or poorly controlled CINV is associated with dehydration, metabolic imbalances, malnutrition, and weight loss leading to frequent hospitalizations and increased use of healthcare resources. Early prevention and active management are essential to reduce the incidence and consequences of CINV and enhance the care of cancer patients.
Significant progress has been made in our understanding of the pathophysiology of CINV, facilitating the development of effective targeted pharmacotherapies including 5-hydroxytryptamine-3 (5-HT3) receptor antagonists and neurokinin-1 (NK-1) receptor antagonists. As many as 70% to 80% of CINV cases can be prevented with appropriate administration of evidence-based antiemetic regimens.
Classification of Chemotherapy-Induced Nausea and Vomiting
CINV is classified into three major categories according to the time of onset: acute, delayed, and anticipatory. In addition, two further categories describe uncontrolled symptoms: breakthrough and refractory CINV.
- ■
Acute emesis occurs within 24 hours of initial administration of chemotherapy with a peak incidence seen at 4 to 6 hours.
- ■
Delayed emesis occurs more than 24 hours to several days after the initial administration of chemotherapy with a peak incidence at 2 to 3 days. Delayed CINV is typically associated with cisplatin; however, the phenomenon has also been noted with cyclophosphamide, carboplatin, doxorubicin, and ifosfamide administration at higher doses.
- ■
Anticipatory emesis precedes drug administration and is believed to be a conditioned response in patients who have experienced significant CINV during previous cycles of treatment. Common triggers for anticipatory CINV include the environment (physician’s office or infusion room), foods, smells, or even cognitive stimuli.
- ■
Breakthrough CINV is nausea and/or vomiting occurring within 120 hours of chemotherapy administration, despite optimal antiemetic prophylaxis based on CINV guidelines. The incidence of breakthrough CINV is 30% to 50% in patients receiving chemotherapy.
- ■
Refractory CINV is the failure to respond to guideline-directed prophylactic antiemetic agents during a previous cycle of treatment, with nausea and vomiting occurring in subsequent chemotherapy cycles.
Risk Factors for the Development of Chemotherapy-Induced Nausea and Vomiting
There are multiple risk factors for the development of CINV. Younger and female patients both have an increased risk of more frequent and severe CINV, whereas patients who are older, male, and those with a history of chronic alcohol consumption tend to be less affected by CINV. Patients with a history of motion sickness and/or pregnancy-related nausea and vomiting have a higher risk of developing CINV. The emetogenicity of the chemotherapeutic agent is the single most important risk factor for development of CINV and is the major criterion for determining the optimal antiemetic prophylaxis regimen. Emetogenicity is a measure of how likely a chemotherapy agent is to cause emesis without antiemetic premedication.
Antineoplastic agents are classified as:
- ■
highly emetogenic (>90% of patients experience CINV)
- ■
moderately emetogenic (30%–90% of patients experience CINV)
- ■
lowly emetogenic (10%–30% of patients experience CINV)
- ■
minimally emetogenic (<10% of patients experience CINV)
Table 6.2 lists important IV and oral chemotherapeutic agents by emetic risk. For certain agents such as cyclophosphamide and doxorubicin, emetogenicity depends on the dose. The risk of developing CINV can also be influenced by the route and frequency of chemotherapy administration. For combination regimens, emetogenic levels are determined by identifying the most emetogenic agent in the combination and then assessing the relative contribution of the other agents.
| Degree of Emetic Risk | Intravenous Agents | Oral Agents |
|---|---|---|
| High (>90%) | Anthracycline + cyclophosphamide Cisplatin Cyclophosphamide >1500 mg/m 2 Dacarbazine | Procarbazine |
| Moderate (30%–90%) | Azacitidine Bendamustine Busulfan Carboplatin Clofarabine Cyclophosphamide <1500 mg/m 2 Cytarabine >1000 mg/m 2 Anthracyclines Ifosfamide Irinotecan Oxaliplatin | Cyclophosphamide Temozolomide Vinorelbine |
| Low (10%–30%) | Cabazitaxel Cytarabine <1000 mg/m 2 Docetaxel Eribulin Etoposide Fluorouracil Gemcitabine Methotrexate Mitomycin Mitoxantrone Paclitaxel Pemetrexed Topotecan Vinflunine | Capecitabine Etoposide Fludarabine Lenalidomide Tegafur-uracil Thalidomide |
| Minimal (<10 %) | Bleomycin Cladribine Fludarabine Pralatrexate Vinca alkaloids | Busulfan Chlorambucil Hydroxyurea Melphalan Methotrexate Pomalidomide 6-Thioguanine |
Pathophysiology of Chemotherapy-Induced Nausea and Vomiting
The pathophysiology of CINV is complex and multifactorial. It involves multiple neurotransmitters, neuroreceptors, and neuronal pathways in the central nervous system (CNS) and peripheral structures. The three main neurotransmitters are serotonin, substance P, and dopamine and the receptors associated with them are 5-HT3, NK-1, and dopamine-2 (D2) receptors, respectively. Other receptors involved include corticosteroid, histamine, cannabinoid, acetylcholine, and opiate receptors, although their roles are not precisely defined.
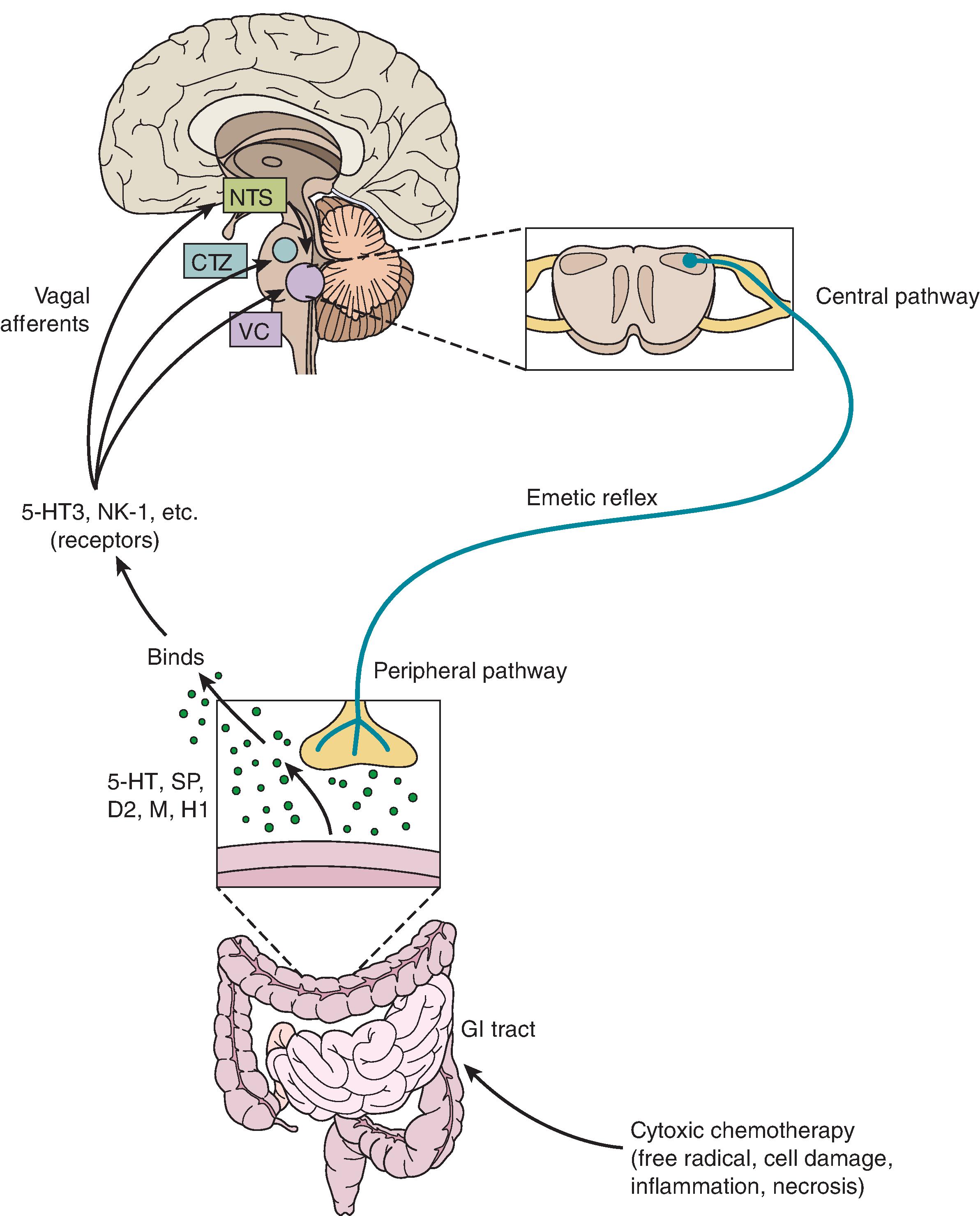
CINV mainly occurs via activation of peripheral and central pathways. The peripheral pathway is mediated via binding of serotonin to 5-HT3 receptors located in the GI tract and is activated within the first 24 hours after chemotherapy administration; it is primarily associated with acute emesis. The central pathway is activated via binding of substance P to NK-1 receptors in the brain and is thought to be predominantly involved in delayed CINV. A simplified illustration of the pathways involved in the pathophysiology of CINV has been depicted in Fig. 6.1 . Chemotherapy can stimulate the enterochromaffin cells lining the digestive tract to release serotonin in response to cell damage. Serotonin binds to 5-HT3 receptors on the nearby vagal afferents in the gut, which in turn causes the transmission of sensory input from the GI tract to the vomiting center in the brain, located in the dorsolateral border of medulla. The vomiting center receives signals from other structures, including the chemotherapy trigger zone (CTZ) in the area postrema, which is lined by 5-HT3 and D2 receptors. Chemotherapy can directly activate the CTZ chemoreceptors because they lack an effective blood–brain barrier. All the sensory signals are consolidated in the vomiting center, resulting in the generation of efferent signals that lead to contraction of the abdominal muscles, the stomach, and the diaphragm, causing subsequent emesis. Each of these neurotransmitters and their corresponding receptors are of primary interest and therapeutic targets for development of antiemetic drugs.
Management and Prevention of Chemotherapy-Induced Nausea and Vomiting
PHARMACOLOGICAL THERAPY FOR CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING
CINV is treated by four major classes of medications: 5-HT3 receptor antagonists, NK-1 receptor antagonists, corticosteroids, D2 receptor antagonists, and Olanzapine has been added to the standard antiemetic guidelines due to its effectiveness in managing CINV.
- 1.
5-HT3 receptor antagonists: These agents act both centrally and peripherally by antagonizing the action of serotonin at 5-HT3 receptors present in the CTZ and the GI tract. Their efficacy in preventing nausea and vomiting induced by moderately emetogenic chemotherapy (MEC) and highly emetogenic chemotherapy (HEC), coupled with a mild side-effect profile, have made them a key component of prophylactic treatment for acute CINV. Ondansetron, granisetron, and palonosetron are US Food and Drug Administration (FDA)–approved for CINV. Palonosetron is the only second-generation 5-HT3 receptor antagonist and has a stronger binding affinity for serotonin receptors and a longer half-life (about 40 hours) compared with first-generation compounds. Initial trials recorded superior efficacy of palonosetron over other 5-HT3 receptor antagonists in terms of complete response, and rates of acute and delayed CINV in patients receiving MEC. , However, subsequent studies demonstrated that all 5-HT3 antagonists have comparable efficacy in preventing CINV and therefore, current guidelines recommend use of any of the available 5-HT3 receptor antagonists, tailored to individual needs. The main side effects of 5-HT3 receptor antagonists are constipation, mild headache, and dizziness. Almost all 5-HT3 receptor antagonists have been associated with asymptomatic electrocardiogram changes, such as prolongation of the PR and QTc intervals and benign ventricular arrhythmias, and they should be used in caution in patients with long QT syndrome. Periodic electrocardiographic monitoring is recommended for patients at high risk such as those with congestive heart failure, electrolyte abnormalities, or bradyarrhythmias, and those treated with concomitant medications that can prolong the QTc interval. Palonosetron is the only 5-HT3 receptor antagonist that does not cause clinically significant QT interval prolongation.
- 2.
NK-1 receptor antagonists: These agents work by blocking substance P activity at NK-1 receptors in the CNS. Examples include the oral NK-1 receptor antagonist, aprepitant, and the IV prodrug fosaprepitant. These drugs have significantly improved the ability to prevent acute and delayed CINV when used in conjunction with both 5-HT3 receptor antagonists and corticosteroids. The efficacy of combining an NK-1 receptor antagonist with a 5-HT3 receptor antagonist and corticosteroid for the prevention of CINV was initially addressed in a meta-analysis of 17 trials, with a total of 8740 patients who received highly or moderately emetogenic chemotherapeutic agents. The three-drug regimen significantly improved rates of complete response (absence of emesis and no need for rescue antiemetics) compared with a two-drug regimen (72% vs. 54%). This led to newer NK-1 antagonists being incorporated into antiemetic guidelines. NEPA is a fixed-dose oral combination of netupitant (highly selective NK-1 receptor antagonist) and palonosetron which is approved for patients receiving highly and moderately emetogenic chemotherapy. Another, NK-1 receptor antagonist, rolapitant, is approved in combination with other antiemetic agents in adults for preventing delayed CINV based on promising results of three phase III trials evaluating rolapitant in patients receiving HEC or MEC. , A number of NK-1 receptor antagonists (aprepitant and fosaprepitant) are inhibitors of the cytochrome P450 3A4 enzyme (CYP3A4), which metabolizes approximately half of all drugs currently on the market and thus caution should be used when coadministering them with oral contraceptives, dexamethasone, warfarin, and other CYP3A4 inducers and inhibitors. Rolapitatant does not inhibit or induce CYP3A4; however, it does have CYP2D6 inhibitory activity. Adverse events associated with NK-1 receptor antagonists include dizziness, anorexia, and diarrhea.
- 3.
Corticosteroids: Corticosteroids are a mainstay of any CINV prophylaxis regimen, but their mechanism of action is not well understood. Several hypotheses have been formulated, such as alteration of cell permeability, inhibition of prostaglandin activity, and activation of glucocorticoid receptors in the nucleus of the solitary tract in the medulla. Dexamethasone and methylprednisolone are the most commonly administered antiemetics. Corticosteroids can be effective when administered as a single agent in patients receiving low emetogenic chemotherapy; however, they are most beneficial when used in combination with 5-HT3 and NK1 receptor antagonists. They have demonstrated effectiveness for both acute and delayed emesis caused by MEC and HEC. Side effects from single or short-term dexamethasone usage are infrequent, but their toxicities for repeated use should not be underestimated. Because NK-1 receptor antagonists, such as aprepitant and netupitant, are moderate inhibitors of CYP3A4, the oral dose of dexamethasone should be reduced by 50% when it is coadministered with NK-1 receptor antagonists.
- 4.
D2 receptor antagonists: D2 receptor antagonists work by competitive blockade of dopamine receptors centrally in the CTZ and vomiting center. A number of different drugs, including droperidol, prochlorperazine, promethazine, and metoclopramide, have demonstrated antiemetic activity. Metoclopramide blocks dopamine receptors centrally in the CTZ, and peripherally in the GI tract, and also has prokinetic effects, thereby increasing gut motility. However, D2 receptor antagonists, can cause extrapyramidal symptoms, restlessness, hypotension, and CNS depression, limiting the use of these drugs, and thus they have been largely replaced by 5-HT3 receptor antagonists due to their superior efficacy and safety. Currently, use of D2 receptor antagonists has been limited to the prevention of nausea from MEC, treatment of breakthrough nausea and vomiting, and in patients with refractory CINV to serotonin blockers.
- 5.
Olanzapine: Olanzapine is a 5-HT2, 5-HT3, and dopamine receptor antagonist and was initially approved as an antipsychotic for use in the treatment of schizophrenia, bipolar disorder, and depression. Various randomized trials have suggested a promising role of olanzapine in the prevention and treatment of acute and delayed CINV. In a phase III study in patients receiving HEC or MEC, olanzapine/5-HT3 receptor antagonist/dexamethasone regimen resulted in superior delayed and overall complete response rates as compared with a 5-HT3 receptor antagonist/dexamethasone regimen. In another phase III trial comparing an olanzapine-based regimen with an aprepitant-based regimen in chemotherapy-naive patients receiving HEC, complete response was observed in 117 patients (97%) in the olanzapine group and 104 patients (87%) in the aprepitant group ( P > .05). The olanzapine regimen resulted in higher complete response rates during the delayed and overall phases (87% acute, 69% delayed/overall) compared with the aprepitant regimen (87% acute, 38% delayed/overall). In the most recent trial, the benefit of adding olanzapine to a triplet 5-HT3 receptor antagonist/apretitant/dexamethasone regimen was evaluated. The percentage of patients with no nausea reported was significantly higher in the olanzapine arm compared with the placebo arm in the acute (74% vs. 45%; P = .002), delayed (42% vs. 25%; P = .002), and overall periods (37% vs. 22%; P = .002). A systematic review of randomized trials has supported the use of olanzapine as a single agent for the treatment of breakthrough CINV for patients who did not receive it prophylactically. In a randomized clinical trial, olanzapine demonstrated superiority over metoclopramide in preventing breakthrough nausea (68% vs. 23%; P < .01) and vomiting (60% vs. 23%; P < .01) for patients receiving HEC who received prior prophylaxis with palonosetron, fosaprepitant, and dexamethasone. Major side effects of olanzapine include drowsiness, fatigue, disturbed sleep, and dry mouth. Olanzapine should not be coadministered with other dopamine receptor antagonists due to increased risk of extrapyramidal symptoms. Overall, the olanzapine-containing regimens appear to be safe, well tolerated, and cost-effective and have efficacy equivalent to that of aprepitant-based regimens. The National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology (ASCO) guidelines have been updated to recommend the use of olanzapine together with 5-HT3 receptor antagonists, dexamethasone, and aprepitant for adults receiving HEC. ,
Antiemetic Regimens Based on Emetogenicity of Chemotherapy
- ■
Antiemetic regimens for highly emetogenic chemotherapy: Patients treated with cisplatin or other highly emetogenic single chemotherapeutic agents should be offered a four-drug combination of a NK1 receptor antagonist, a 5-HT3 receptor antagonist, dexamethasone, and olanzapine on day 1 of treatment. Dexamethasone and olanzapine are recommended to be continued on days 2 to 4 ( Table 6.3 ). Alternatively, three-drug regimens including single doses of a 5-HT3 receptor antagonist, dexamethasone, and an NK1 receptor antagonist can also be given for prevention of CINV for HEC. For patients receiving anthracycline/cyclophosphamide (AC) combination chemotherapy, a four-drug combination of NK-1 receptor antagonist, 5-HT receptor antagonist, dexamethasone, and olanzapine is recommended prior to the start of cytotoxic treatment (day 1) and only olanzapine is continued on days 2 to 4. Dexamethasone is not recommended on days 2 to 4 due to limited data supporting its benefit beyond day 1. Alternatively, a three-drug regimen including single doses of a 5-HT3 receptor antagonist, dexamethasone, and an NK1 receptor antagonist can also be given for prevention of CINV in patients receiving AC combination chemotherapy.
TABLE 6.3■
Antiemetic Dosing for Adults Receiving Chemotherapy
Regimen
Drugs
Dose on Day 1
Dose on Days 2, 3, 4
Highly Emetogenic Chemotherapy
4-Drug combination regimen (NK-1 RA, 5-HT3 RA, dexamethasone, olanzapine)
NK-1 RA (choose one):
Aprepitant
Fosaprepitant
Rolapitant
NEPA
125 mg PO
150 mg IV once
180 mg PO once
(300 mg netupitant/0.5 mg palonosetron PO combination) once
80 mg oral on days 2, 3
5-HT3 RA (choose one):
Granisetron
Ondansetron
Palonosetron
2 mg PO or 1 mg or 0.01 mg/kg IV or
1 transdermal patch or 10 mg sc
8 mg PO twice daily or 8 mg or 0.15 mg/kg IV once
0.50 mg oral or 0.25 mg IV once
Dexamethasone
12 mg PO/IV once
8 mg PO/IV daily on days 2, 3, 4
Olanzapine
10 mg PO
10 mg PO daily on days 2, 3, 4
3-Drug NK-1 RA based combination regimen
NK-1 RA (choose one):
Aprepitant
Fosaprepitant
Rolapitant
NEPA
125 mg PO once
150 mg IV once
180 mg PO once
(300 mg netupitant/0.5 mg palonosetron PO combination) once
80 mg PO daily on days 2, 3
5-HT3 RA (choose one):
Granisetron
Ondansetron
Palonosetron
2 mg PO or 1 mg or 0.01 mg/kg IV or
1 transdermal patch or 10 mg sc
8 mg PO twice daily or 8 mg or 0.15 mg/kg IV once
0.50 mg oral or 0.25 mg IV once
Dexamethasone
12 mg PO/IV once
8 mg PO/IV daily on days 2, 3, 4
Moderately Emetogenic Chemotherapy
Carboplatin based: 3-drug combination regimen (NK-1 RA + 5-HT3 RA + dexamethasone)
NK-1 RA (choose one):
Aprepitant
Fosaprepitant
Rolapitant
NEPA
125 mg PO
150 mg IV once
180 mg PO once
(300 mg netupitant/0.5 mg palonosetron PO combination) once
80 mg PO daily on days 2, 3
5-HT3 RA (choose one):
Granisetron
Ondansetron
Palonosetron
2 mg PO or 1 mg or 0.01 mg/kg IV or
1 transdermal patch or 10 mg sc
8 mg PO twice daily or 8 mg or 0.15 mg/kg IV once
0.50 mg oral or 0.25 mg IV once
Dexamethasone
12 mg PO/IV once
Non-carboplatin based: 2-drug combination regimen (5-HT3 RA + dexamethasone)
5-HT3 RA (choose one):
Granisetron
Ondansetron
Palonosetron
2 mg PO or 1 mg or 0.01 mg/kg IV or
1 transdermal patch or 10 mg sc
8 mg PO twice daily or 8 mg or 0.15 mg/kg IV once
0.25 mg IV once
Dexamethasone
8 mg PO/IV once
8 mg PO/IV on day 3
Low Emetogenic Chemotherapy
Single agent dexamethasone
Dexamethasone
8 mg PO/IV once
Single agent 5-HT3 RA
5-HT3 RA (choose one):
Granisetron
Ondansetron
Palonosetron
2 mg PO or 1 mg or 0.01 mg/kg IV or
1 transdermal patch or 10 mg sc
8 mg PO twice daily or 8 mg or 0.15 mg/kg IV once
0.25 mg IV once
Single agent dopamine RA
Dopamine RA (choose one):
Metoclopramide
Prochlorperazine
10–20 mg PO/IV once
10 mg PO/IV once
Minimally Emetogenic Chemotherapy
No routine prophylactic antiemetics

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree