Chapter 7
Gastroenterology
Sarah Macdonald
Introduction
Gastroenterology is one of the most interesting and challenging areas in paediatric dietetics. The medical conditions encountered are diverse and require an understanding of normal gastrointestinal (GI) function before correct dietetic advice can be given. Problems as varied as diarrhoea, constipation and GI dysmotility can affect normal intake and absorption of nutrients. Manipulation of feeds and diet is often the primary treatment for the underlying condition and carers need careful explanation of the principles of the feed and diet prescribed.
Nutritional requirements
Nutritional requirements vary according to the underlying disorder. Normal requirements for most nutrients will suffice for GI disorders that do not result in malabsorption, with additional energy and protein required for catch-up growth.
When malabsorption is present requirements for all nutrients are raised to allow for stool losses particularly fluid, energy, protein and electrolytes. Most infants will have high to very high requirements. Table 7.1 can be used as a guide for requirements for infants with malabsorption who are fed enterally and are based on actual rather than expected weight. For all the clinical conditions described the assessment and monitoring of the child’s nutritional status is paramount. Anthropometric measurements should be plotted serially on appropriate growth charts. In conditions resulting in malabsorption, or in those requiring a very restrictive diet, iron indices, trace elements, vitamins (particularly fat soluble vitamins and B12) and urinary sodium levels should be monitored with additional supplements prescribed as necessary.
Table 7.1 Suggested requirements for infants with malabsorption, per day
| Energy | High | 130–150 kcal/kg (540–630 kJ/kg) |
| Very high | 150–220 kcal/kg (630–900 kJ/kg) | |
| Protein | High | 3–4 g/kg |
| Very high | Maximum 6 g/kg | |
| Sodium | High | 3.0 mmol/kg |
| Potassium | Very high | 4.5 mmol/kg |
| Fluid | High | 180–220 mL/kg |
Acute diarrhoea—fluid and dietary therapy
Acute diarrhoea remains one of the leading causes of childhood morbidity and mortality in developing nations, with an estimated 5–18 million deaths attributed to this cause each year. In industrial nations the mortality rate is much lower. Infants and children are particularly vulnerable to the effects of acute diarrhoea because of their greater relative fluid requirements and their susceptibility to faecal-oral agents.
The causative mechanisms in the GI tract are
- increased secretion
- decreased absorption
Often these coexist to produce an increased fluid load that exceeds the colonic absorptive capacity, resulting in diarrhoea. Both viral and bacterial pathogens can affect the gut in this way. Diarrhoea and vomiting can also be due to other infections (such as urinary tract infections), food hypersensitivity or surgical causes which should be considered on presentation.
Transport of glucose and amino acids is an active process and requires the presence of a sodium gradient across the brush border membrane maintained by the Na+-K+ ATPase pump. The movement of water in the gut is a passive event driven by the movement of solute. The regulation of electrolyte transport is controlled by several mediators and inhibition of these pathways results in poor absorption and active chloride secretion into the gut.
In infective diarrhoea the decreased absorption seen is not necessarily due to reduced villous size. With increased cell loss immature epithelial cells replace fully differentiated, mature absorptive cells. These cells demonstrate defective electrolyte and nutrient transport and functional impairment may be severe. This situation is worsened by cycles of fasting and starvation commonly seen in infants and children with acute diarrhoea in developing countries.
Acute gastroenteritis is defined as a decrease in stool consistency (loose or liquid) and/or an increase in the frequency of bowel actions with or without fever or vomiting. The diarrhoea should not last longer than 14 days. In Europe the incidence ranges from 0.5 to 1.9 episodes per child per year in children younger than 3 years, with rotavirus being the most frequent infective agent [1].
Oral rehydration solutions
Oral rehydration therapy is used to correct dehydration and maintain hydration. The sodium–glucose coupled transport mechanism stimulates water and electrolyte transport. This process is preserved in acute diarrhoeal disorders and first line therapy for managing acute gastroenteritis in children should utilise this mechanism. If the child cannot drink sufficiently then a nasogastric tube should be passed to ensure that the oral rehydration solution (ORS) can be given. Enteral rehydration is associated with significantly fewer major adverse events and a shorter hospital stay compared with intravenous (IV) therapy and is successful in most children [1].
Specific recommendations for the composition of ORS for European children were published by the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) in 1992 [2]:
- carbohydrate should be present as either glucose or glucose polymer at concentrations between 74 and 111 mmol/L
- ORS should contain 60 mmol/L sodium, compared with 75 mmol/L recommended by the World Health Organization (WHO) and United Nations Children’s Fund (UNICEF) in developing countries, to minimise the risk of hypernatraemia
- potassium should be added to replace stool losses
- osmolality should be low (200–250 mOsm/kg H2O) to ensure optimal water absorption
Systematic reviews have confirmed that this is still the best composition of ORS to use in children admitted to hospital with diarrhoea [3]. However, there may be some advantage in using polymer based ORS for all cause diarrhoea and in diarrhoea caused by cholera [4]. The ORS available in the UK are summarised in Table 7.2.
Table 7.2 Oral rehydration solutions (mmol/L)
| Na+ | K+ | Cl− | CHO | |
| Dioralyte* | 60 | 20 | 60 | 90 |
| (Sanofi-Aventis) | (glucose) | |||
| Electrolade* | 50 | 20 | 40 | 111 |
| (Actavis) | (glucose) | |||
| Dioralyte Relief* | 60 | 20 | 50 | 30 g |
| (Sanofi-Aventis) | (rice starch) | |||
| WHO formulation | ||||
| Oral Rehydration Salts | 75 | 20 | 65 | 75 |
| (glucose) |
Flavoured preparations are not suitable for young infants.
* Reconstitute 1 sachet with 200 mL water.
Feeding during acute diarrhoea
For many years it was common practice to stop feeds during diarrhoeal episodes. It was thought that decreased lactase activity, chiefly associated with rotavirus gastroenteritis, would cause lactose malabsorption if milk feeds were introduced too early and that food proteins could be transported across an impaired mucosal barrier and cause sensitisation [5]. Consequently bottle fed infants with gastroenteritis were fed ORS alone for 24 hours followed by the introduction of dilute feeds. This advice resulted in a reduced nutritional intake at a time when requirements were increased due to infection [6].
A meta-analysis of randomised clinical trials published in 1994 showed that the routine dilution of milk feeds and use of lactose free formula was not justified in the treatment of infants and children with acute diarrhoea [7]. A multicentre European study showed that the complete resumption of a child’s normal feeding after 4 hours of rehydration with ORS led to a significantly greater weight gain during hospitalisation and did not result in worsening or prolonged symptoms [8]. A recent meta-analysis has shown the same outcome [9]. This is especially important in developing countries where children may already be malnourished.
ESPGHAN has published recommendations that management of gastroenteritis should consist of oral rehydration with a low osmolar ORS for 4 hours (100 mL/kg over 4–6 hours in moderately dehydrated patients), followed by resumption of normal feeding [10]. Formula dilution and gradual reintroduction of feeding is not indicated. Supplementing the usual feeds with ORS (10 mL/kg/liquid stool) can prevent further dehydration. Breast feeding should be continued at all times with supplementation of ORS.
Use of lactose free formula
There is no evidence to support the use of reduced lactose or cow’s milk protein free formulas in infants and children with acute diarrhoea, even if the infective agent is rotavirus. A very small minority of patients who show signs of feed intolerance (defined as worsening of diarrhoea with acidic stools containing >0.5% reducing substances) may need the temporary use of a low lactose formula (Table 7.3). These have been shown to support normal growth in infancy [11].
Table 7.3 Low lactose, cow’s milk protein based formulas
| Galactomin 17 (Scientific Hospital Supplies) |
| Enfamil O-Lac (Mead Johnson) |
| SMA LF (SMA Nutrition) |
For lactose content see Table 18.9.
Congenital chloride losing diarrhoea
This is a selective defect in intestinal chloride transport in the ileum and colon which is inherited as an autosomal recessive trait. Lifelong secretory diarrhoea occurs with high chloride concentrations. It has been reported in most populations including the UK; however, it is most commonly seen in Finland, the Arabian Gulf and Poland [12].
In the past it generally resulted in severe lethal dehydration. Watery diarrhoea is present from birth but often goes unnoticed as the fluid in the nappy is thought to be urine. Dehydration occurs rapidly followed by disturbances in electrolyte concentration causing hypokalemia and hypochloraemia with mild metabolic alkalosis.
Treatment
As the intestinal defect cannot be corrected treatment requires replacement of the diarrhoeal losses of chloride, sodium and water. Initially this may need to be given IV but this should gradually be changed to the oral route. Insufficient salt substitution causes chronic dehydration, salt depletion and activation of the renin-aldosterone system which may lead to chronic kidney disease. Dietary manipulation is not required in this disorder other than to ensure a normal intake for age in conjunction with the prescribed electrolyte and fluid therapy.
Food allergy in gastroenterology
It is thought that the relatively high incidence of adverse reactions to food proteins seen in infancy is the result of immaturity of local and systemic immune systems, often in association with increased gut permeability to large molecules. Allergic sensitisation involves a failure to develop oral tolerance, an immunologic response induced by tolerogenic food proteins in contact with the gut associated lymphoid tissue. One common cause of this is the postenteritis syndrome where a loss of barrier function and the breakdown of normal immune tolerance follows an enteric infection. Deficiency of immunoglobulin A (IgA), which is involved in the immune defence of mucosal surfaces, is a common associated finding in allergic infants.
Food allergy may broadly be classified as either antibody mediated, e.g. IgE mediated (immediate GI hypersensitivity and oral allergy syndrome), or cell mediated, e.g. T cell mediated (dietary protein enteropathy, protein induced enterocolitis and proctitis) [13, 14]. In some patients both mechanisms can coexist (eosinophilic oesophagitis and gastroenteritis). Pathological inflammatory changes can be seen in the GI tract. Cells and mediators of the immune system such as eosinophils (a type of white blood cell) and lymphocytes can be found in biopsies of inflamed sites. Allergic reactions can affect GI secretion, absorption (with or without mucosal damage) and motility. Interactions between the allergic cells and the mucosal nervous system are important in mediating alterations in secretion and motility. Both IL-5 (a Th2 produced cytokine) and the chemokine eotaxin play a role in allergic responses that can present as delayed gastric emptying, gastro-oesophageal reflux and constipation [15]. GI conditions caused by allergic reactions to dietary proteins are summarised in Table 7.4. Often in the clinical setting dietary manipulations are used to treat symptoms before any formal investigations are carried out. An algorithm of suggested management is given in Fig. 7.1.
Table 7.4 Gastrointestinal disorders that can be caused by allergy to dietary proteins
| Oral allergy syndrome |
| Eosinophilic oesophagitis |
| Eosinophilic gastroenteropathy |
| Food protein induced enterocolitis syndrome (FPIES) |
| Eosinophilic colitis |
| Enteropathy |
| Proctocolitis |
| Constipation |
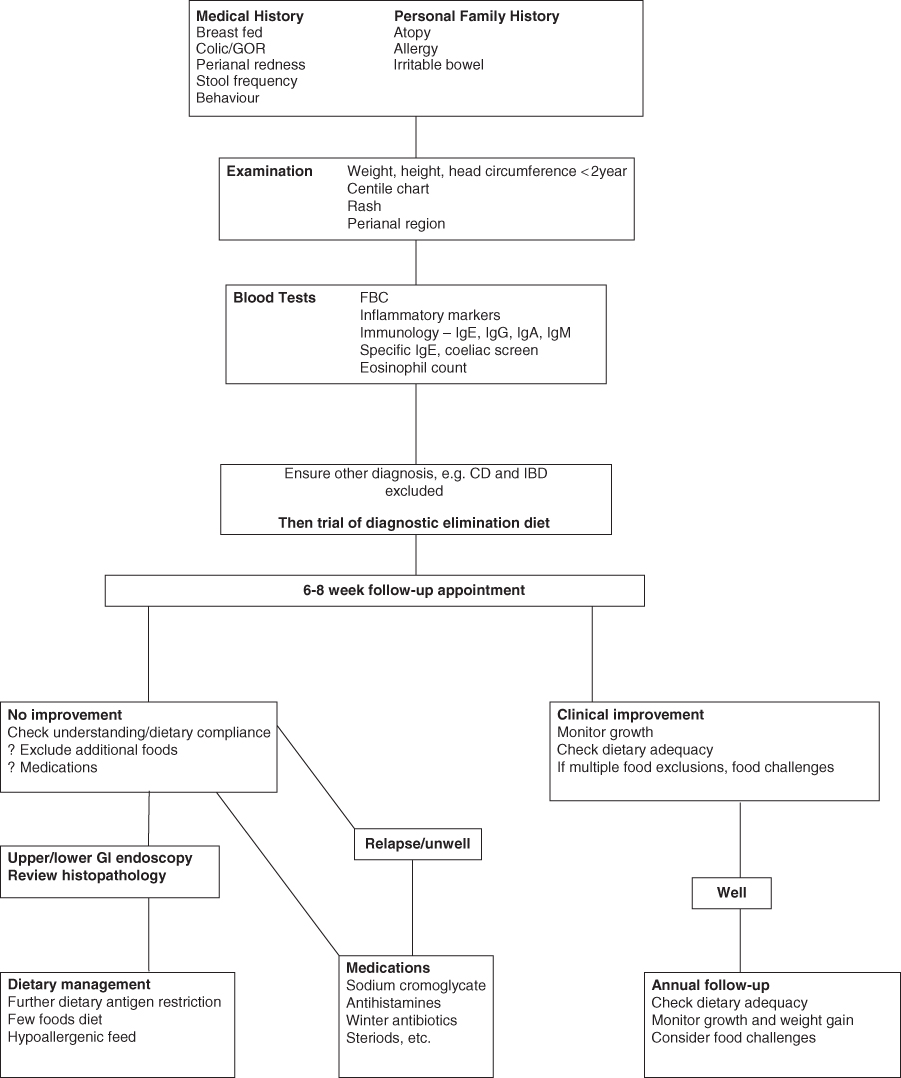
Figure 7.1 Suggested management of food allergy in gastroenterology. CD, coeliac disease; FBC, full blood count; GI, gastrointestinal; GOR, gastro-oesophageal reflux; IBD, inflammatory bowel disease; Ig, immunoglobulin.
Although the most common foods to cause GI food allergy problems are cow’s milk, egg, wheat and soya, any food ingested could be allergenic [16, 17]. The current tests available (skin prick tests, patch tests and specific IgE) are of limited use in identifying food allergens causing GI disease. The prescribed exclusion diet is usually based on an underlying first degree family history of atopy (hay fever/allergic rhinitis, asthma, eczema), allergies and organ specific autoimmunity combined with the age at presentation of symptoms and food intake at that time. Sometimes a number of dietary manipulations need to be tried before the correct dietary restriction for the individual is achieved. In the presence of multiple food allergies a few foods diet (p. 314) or exclusive use of a hypoallergenic feed may be needed with subsequent single food introductions to identify the causative food allergens.
Exclusion diets are difficult to manage at home and are expensive. Selection of suitable patients is important. Use of anti-allergic or anti-inflammatory drugs as a therapeutic alternative to dietary restriction should be considered in situations where the family will not cope with a strict exclusion diet.
When multiple foods are excluded from the diet it is important to sequentially challenge the excluded foods (p. 315) to identify those the child is reacting to in order to avoid over-restricting the diet.
Non-IgE mediated gastrointestinal hypersensitivities
Eosinophilic gastrointestinal diseases
Eosinophils are customary inhabitants and key effector cells of the innate immune system within the GI tract. They protect against parasitic infections, the rate of which has decreased markedly in the developed world. It is thought that hypersensitivity reactions to allergens may now be the driving force for recruitment and activation of gut eosinophils. When activated they release multiple cytotoxic agents and immunomodulatory cytokines resulting in local inflammation and tissue damage [18].
Eosinophilic gastrointestinal diseases (EGID) are a heterogeneous group of diseases (eosinophilic oesophagitis, eosinophilic gastroenteritis, eosinophilic colitis) characterised by GI symptoms and increased eosinophils in the absence of other causes. Apart from eosinophilic oesophagitis the diagnosis of these disorders is unclear due to the uncertainty as to the normal number of eosinophils seen in different parts of the GI tract and their distribution within the mucosa [19].The latter may differ with geographical environment.
EGID are classified based on the location of the inflammatory response, even though their symptoms, prognosis and treatment vary considerably. In view of the close relationship between food allergies and some EGID, controlling antigen exposure is one of the most widely used strategies. In one study 80% of patients with EGID were found to have coexisting atopy and anaphylaxis to foods was present in a significant number [20].
Eosinophilic oesophagitis
This is the most common and best described of the EGID and is seen in both children and adults. It is a chronic immune/antigen mediated disease characterised clinically with symptoms related to oesophageal dysfunction and histologically by eosinophil predominant inflammation. There are high rates of concurrent asthma, allergic rhinitis, eczema and food allergy/anaphylaxis. It is more common in boys. Young children typically present with symptoms of gastro-oesophageal disease (GORD), food refusal or growth faltering whilst adolescents tend to present with dysphagia [21]. Complications include food impaction, oesophageal stricture formation and perforation. Serum IgE and skin prick tests for immediate type food allergy are warranted to identify comorbid food induced allergic disease, but these tests alone are not sufficient to make the diagnosis of food allergy driven eosinophilic oesophagitis. First line therapies must include the use of protein pump inhibitors, as oesophageal eosinophilia can be secondary to GORD. If symptoms fail to improve then dietary therapy should be tried as sole treatment or in conjunction with topical swallowed corticosteroids (steroids that are usually inhaled but are taken orally) [22].
Dietary therapy has been shown to be an effective treatment when assessing the histopathology of patients and three different regimens have been used. Complete amino acid based feeds have been shown to induce remission in 96% of patients, the majority of whom needed a nasogastric tube to complete the treatment; the trial of feeds is followed by an extended period of food reintroductions to identify specific allergens [23]. Dietary restrictions based on allergy testing or the exclusion of the most likely food antigens have also been used. The former was successful in 69% of patients [24].The latter (excluding cow’s milk, soy, wheat, egg, peanut and seafood) has demonstrated a 74% success rate [25]. A recent retrospective case series of patients treated with one of these three elimination diets showed the greatest efficacy with complete amino acid based (elemental) feeds with 96% remission. Empirical removal of the most common allergens from the diet was as successful as a directed diet based on skin prick tests and patch testing alone (remission rates of 81% and 65%); however, the treatment chosen was not randomised and depended on negotiations with the family [26].
Consensus recommendations suggest that the specific therapy prescribed should take into account the family lifestyle, adherence to therapy and resources [22, 27]. Multiple food exclusions are difficult for the family to manage and expose the child to the dangers of dietary inadequacy, so careful monitoring is required with amino acid based supplemental feeds or vitamin and mineral supplements as needed. Once the disease is in remission foods should be reintroduced sequentially with careful monitoring so that only the foods the child is allergic to continue to be excluded. In one study cow’s milk was found to be the most common allergen followed by wheat, egg and soy [28].
Eosinophilic gastroenteritis
Eosinophilic gastroenteritis is a chronic inflammatory disorder of the GI tract characterised by eosinophil accumulation in the mucosa or in deeper layers of the GI wall. Symptoms often mimic those of inflammatory bowel disease or irritable bowel syndrome. Under healthy conditions the stomach and the small intestine show detectable baseline numbers of eosinophils but their number is increased in eosinophilic gastroenteritis. Symptoms vary according to the site affected and include pain, dysmotility, nausea, vomiting, diarrhoea, blood loss, malabsorption, protein losing enteropathy and faltering growth [29].
There are no consensus guidelines on which to base therapies due to the rarity of this diagnosis and depends on whether associated food allergy and food allergens can be confirmed. Four food exclusion diets (milk, egg, wheat and soya free), few foods diets or exclusive elemental (amino acid based) enteral feeds can be tried [30]. If there is a limited response to these therapies then medical treatment is unavoidable [31].
Eosinophilic colitis
Primary eosinophilic colitis (EC), also known as allergic colitis of infancy or dietary protein induced proctocolitis, is thought to be non-IgE based (with skin prick tests usually negative).The exact immunological mechanism is not fully understood. This disease entity is predominantly a disorder of infants younger than 3 years. The classical presentation is of blood streaked stools in an otherwise healthy infant; however, it can also be seen in older children and adolescents where it may present with abdominal pain, anorexia and weight loss. Endoscopic findings include increased eosinophils and lymphonodular hyperplasia. Elimination of the causal protein is usually followed by resolution of symptoms [32]. In EC the most common causal protein is cow’s milk and, as a large number of infants with this disorder are breast fed, this necessitates a maternal exclusion diet [30]. In one series of 95 breast fed infants with EC dietary exclusions were successful in 89% of cases. Of the remainder, seven responded to an extensively hydrolysed protein formula (EHF) and the remaining 4 (all of whom had eczema) needed an amino acid based formula (AAF) [33]. Prognosis of EC that develops in infancy is good; however, for young adults the natural history is that of a more chronic disease [34].
Food protein induced enterocolitis syndrome
Food protein induced enterocolitis syndrome (FPIES) is also a non-IgE mediated food hypersensitivity characterised by GI symptoms and a systemic inflammatory response in young infants. This can be chronic when the food is eaten regularly or acute when the causative food is ingested intermittently. Due to the atypical presentation and lack of diagnostic tests FPIES is often misdiagnosed initially and surgical, septic or viral causes investigated. Vomiting is the main feature in all cases with 75% of infants appearing acutely ill and 15%–20% developing hypotension. Local inflammation has been demonstrated on biopsy and it is thought that this leads to increased gut permeability and fluid shifts resulting in emesis, diarrhoea and lethargy.
The most common allergens are cow’s milk, soy and rice although a wide array of other foods have been implicated. Proposed diagnostic criteria are symptoms occurring before 9 months of age; repeated exposure to causative foods elicits GI symptoms without an alternative (including IgE mediated) cause; removal of specific foods results in symptom resolution; re-exposure elicits symptoms within 4 hours. Infants frequently react to more than one food [35]. In an American review where cow’s milk was the causative food, 90% of the patients had recovered by the age of 3 years. No concomitant reaction to soy was detected in this population [36]. In a similar Australian review a wider range of trigger foods were identified (rice, soy, cow’s milk, meats, vegetables and fruits, oats and fish). They also found high rates of resolution for the two most common food triggers before the age of 3 years. Children frequently experienced multiple episodes of FPIES before the correct diagnosis was made [37].
Exclusion of cow’s milk protein
Cow’s milk is the most common food to cause a reaction in infants and the incidence of cow’s milk protein allergy reported in developed countries is between 1.9% and 4.9% with 32%–60% cases developing GI symptoms [38]; 0.5% of breast fed infants are reported to be food allergic or intolerant, reacting to exogenous food proteins secreted into the mother’s milk. When an alternative infant formula is tried it is necessary to persist with this formula for a reasonable length of time, observing symptoms carefully before abandoning it in favour of a different feed. Delayed reactions to dietary proteins can occur several days after their ingestion.
Prognosis is good with remission in approximately 80% of children by 4 years of age with some evidence that children with delayed reactions become tolerant faster than those with immediate reactions [38].
Alternative infant formulas
It is vital that an infant is given a nutritionally complete milk substitute to replace a cow’s milk protein based formula. In breast fed infants the mother’s diet needs to be modified by the removal of cow’s milk and any other foods allergenic to the infant. Care needs to be taken to ensure that the maternal diet continues to include adequate amounts of calcium, vitamin D, energy, protein and fluid. It has been found that breast fed infants can be sensitised to multiple allergens including egg, soy, wheat and fish [39]. ESPGHAN recommends that the dietary trial should be continued for up to 14 days if delayed GI reactions are suspected. If there is no improvement then the child should be further evaluated. If multiple food allergies are suspected then an AAF or EHF can be tried. The mother should be encouraged to express her milk whilst the clinical response to the hypoallergenic feed is evaluated [40].
Mammalian milks
Mammalian milk is not suitable to be used as an infant feed without modification due to its high renal solute load and inadequate vitamin and mineral content. The proteins in goat’s and sheep’s milks share antigenic cross-reactivity with cow’s milk proteins [38, 41]. Infant formulas based on goat’s milk are not recommended for use in GI food intolerances [42].
Soy protein based formulas
A soy protein based formula was used for the first time in 1929 to feed infants with cow’s milk protein allergy. Today these feeds are based on soy protein isolate supplemented with L-methionine to give a suitable amino acid profile for infants. They are lactose free with glucose polymer as the most common source of carbohydrate. The fat is a mixture of vegetable oils that provide long chain fatty acids, including essential fatty acids. Feeding modern soy formulas to infants is associated with normal growth, protein status and bone mineralisation [43].
However, in the UK the use of soy formulas in infants under the age of 6 months has been advised against by the Chief Medical Officer, unless there is a specific medical indication, due to their high phytoestrogen content [44]. There appears to be less risk to the infant after 6 months of age as the amount of isoflavones per kilogram body weight is reduced as dependence on formula as a source of nutrition decreases. The infant’s potentially vulnerable organ systems are likely to have matured by that age. The Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment is reviewing more recent research in this area.
Soy protein has a very large molecular weight and after digestion can generate a large number of potential allergens. IgE mediated soy allergy is common, affecting 0.4% children in the USA [45]. Severe GI reactions to soy protein formula have been described for more than 30 years and include enteropathy, enterocolitis and proctitis. In FPIES caused by cow’s milk protein 30%–64% had concomitant soy induced enterocolitis which responded to the use of EHF. It is suggested that an intestinal mucosa damaged by cow’s milk protein allows increased uptake and increased immunological reaction to soy protein. A reported 60% of infants with cow’s milk protein induced enterocolitis are equally sensitive to soy [46]. The American Academy of Pediatrics state that soy formulas are not recommended in the management of cow’s milk protein enteropathy or enterocolitis; however, most children can resume soy protein consumption safely after 5 years of age [43]. EPSGHAN concludes that the use of EHF (or AAF if the hydrolysate is not tolerated) should be preferred to soy protein feeds and the latter should not be used before the age of 6 months. If soy formulas are used for therapeutic use after the age of 6 months due to their lower cost and better acceptance, tolerance to soy protein should first be established by clinical challenge [46].
Older infants with documented IgE mediated allergy to cow’s milk protein can do well on soy protein based formula [47–49]. In other GI manifestations of possible cow’s milk allergy, such as constipation, soy feeds can be tried. In one of the first papers looking at cow’s milk allergy and chronic constipation 68% of the children responded to soy milk. Soy formula has the benefit of being at least half the cost of EHF and is much more palatable. Prospective studies looking at the incidence of soy allergy in patients with GI conditions are required. Soy infant formulas available in the UK are summarised in Table 7.5.
Table 7.5 Composition of soy infant formulas, per 100 mL
| Energy | |||||||
| Dilution | CHO | Protein | Fat | Osmolality | |||
| (%) | (kcal) | (kJ) | (g) | (g) | (g) | (mOsm/kg H2O) | |
| InfaSoy | |||||||
| (Cow & Gate) | 12.8 | 66 | 275 | 7 | 1.6 | 3.5 | 150 |
| Wysoy | |||||||
| (SMA Nutrition) | 13.2 | 67 | 280 | 6.9 | 1.8 | 3.6 | 204 |
Feeds based on protein hydrolysates
Infants with cow’s milk allergy and proven or suspected soy intolerance need an alternative formula. The allergenicity or antigenicity of a particular protein is a function of the amino acid sequences present and the configuration of the molecule. An epitope is the area of a peptide chain capable of stimulating antibody production. During the manufacture of a hydrolysate the protein is denatured by heat treatment and hydrolysed by proteolytic enzymes leaving small peptides and free amino acids. The enzymes are then inactivated by heat and, along with residual large peptides, are removed by filtration [50].
The proteins used to make a hydrolysate vary and production methods also differ between manufacturers. The profile of peptide chain lengths between different feeds will not be identical, even when the initial protein is the same.
Potential problems with hydrolysate formulas
Despite the rigorous conditions employed in the manufacture of these feeds there are still potential sequential epitopes present that can be ‘recognised’ by sensitive infants. Extensively hydrolysed protein based feeds vary considerably in their molecular weight profile and hence in their residual allergenic activity (Table 14.7). Feeds with peptides >1500 Da have been demonstrated to have residual allergenic activity [51, 52]. The degree of hydrolysis does not predict the immunogenic or the allergenic effects in the recipient infant. It has been recommended that dietary products for treatment of cow’s milk protein allergy in infants should be tolerated by at least 90% of infants with documented cow’s milk allergy [42]. In instances where an infant is not malnourished and fails to tolerate one EHF, a second EHF from a different protein source can be tried.
Table 7.6 shows the composition of EHF available in the UK. Feed choice may be influenced by
- palatability, which is affected by the presence of bitter peptides. This is particularly important in infants older than 3 months of age
- coexisting fat malabsorption, where a feed with some of the fat as medium chain triglycerides (MCT) may be indicated
- cost, some hydrolysates being twice as expensive as others
- religion and culture, where parents do not wish their children to be given products derived from pork
Table 7.6 Extensively hydrolysed infant formulas, per 100 mL
| Energy | |||||||||
| Dilution | CHO | Protein | Fat | Na | K | Osmolality | |||
| (%) | (kcal) | (kJ) | (g)* | (g) | (g)† | (mmol) | (mmol) | (mOsm/kg H2O) | |
| Casein | |||||||||
| Nutramigen Lipil 1 | 13.6 | 68 | 280 | 7.5 | 1.9 | 3.4 | 1.4 | 2.1 | 280 |
| (Mead Johnson) | |||||||||
| Nutramigen Lipil 2‡ | 14.6 | 68 | 285 | 8.6 | 1.7 | 2.9 | 1.1 | 2.1 | 340 |
| (Mead Johnson) | 17% | ||||||||
| fructose | |||||||||
| Pregestimil Lipil | 13.5 | 68 | 280 | 6.9 | 1.9 | 3.8 | 1.3 | 1.9 | 280 |
| (Mead Johnson) | (55%) | ||||||||
| Similac Alimentum | 12.9 | 68 | 283 | 6.6 | 1.9 | 3.8 | 1.3 | 1.8 | 274 |
| (Abbott) | 22% | (33%) | |||||||
| sucrose | |||||||||
| Whey | |||||||||
| Pepti-Junior | 12.8 | 66 | 275 | 6.8 | 1.8 | 3.5 | 0.8 | 1.7 | 210 |
| (Cow & Gate) | trace | (50%) | |||||||
| lactose | |||||||||
| Aptamil Pepti 1 | 13.6 | 67 | 280 | 7.1 | 1.6 | 3.1 | 0.9 | 1.9 | 280 |
| (Milupa ) | 37% | ||||||||
| lactose | |||||||||
| Aptamil Pepti 2‡ | 14.3 | 68 | 285 | 8 | 1.6 | 3.1 | 1.1 | 2.0 | 290 |
| (Milupa) | 36% | ||||||||
| lactose | |||||||||
| Althera | 13.2 | 67 | 280 | 7.3 | 1.7 | 3.4 | 0.8 | 1.8 | 335 |
| (Vitaflo) | 52% | ||||||||
| lactose | |||||||||
| Infatrini Peptisorb§ | – | 100 | 420 | 10.3 | 2.6 | 5.4 | 1.4 | 2.8 | 350 |
| (Nutricia) | trace | (50%) | |||||||
| lactose | |||||||||
| Pork and soya collagen | |||||||||
| Pepdite | 15 | 71 | 297 | 7.8 | 2.1 | 3.5 | 1.5 | 1.5 | 237 |
| (SHS) | (5%) | ||||||||
| MCT Pepdite | |||||||||
| (SHS) | 15 | 68 | 286 | 8.8 | 2.0 | 2.7 | 1.5 | 1.5 | 290 |
| (75%) | |||||||||
SHS, Scientific Hospital Supplies.
* Carbohydrate is present as glucose polymers unless otherwise stated.
† Figures in parentheses indicate the percentage of fat present as medium chain triglycerides (MCT).
‡ Suitable from 6 months of age.
§ Liquid feed, designed for infants <9 kg body weight requiring a nutrient dense feed. Not suitable for cow’s milk allergy.
Introduction of hydrolysate formulas
Hydrolysed protein formulas should be introduced slowly to infants with severe GI symptoms as they have a higher osmolality than normal infant formulas. Feeds containing a high percentage of MCT should also be introduced gradually to ensure tolerance. If the diarrhoea is very severe then it may be necessary to introduce quarter strength feeds, grading up to full strength feeds over 4 days. If severe diarrhoea is present in an older infant it is preferable to stop all solids while a new feed is being introduced to assess tolerance.
In an outpatient setting, where symptoms may be less severe, full strength formula can usually be introduced from the outset. In infants older than 6 months there may be an advantage in initially mixing 1 part EHF to 3 parts their usual formula to slowly introduce the new taste and encourage acceptance, increasing the proportion of EHF over the next 3 days. Milk shake flavourings at 2%–4% concentration (2–4 g per 100 mL) can also be used in this age group if sucrose is not contraindicated.
If an infant refuses to drink the EHF a nasogastric tube needs to be passed to ensure adequate volumes are taken. Where faltering growth coexists, feeds can be fortified in the usual manner by increasing formula concentration or the judicial use of carbohydrate and fat supplements [53] (see Table 1.18). All changes should be made slowly to ensure they are tolerated.
Pepti-Junior, Aptamil Pepti 1 and Althera have sodium contents similar to standard infant formula which may be insufficient for an infant with increased stool losses. Low urinary sodium concentation (<50 mmol/L) alongside a normal plasma sodium concentration may indicate sodium depletion and supplementation with sodium chloride may be required.
Amino acid based infant formulas
Only pure amino acid mixtures are considered to be non-allergenic as there are no peptide chains present to act as epitopes. In infants who fail to tolerate an EHF this is the next logical step, so long as there is not a coexisting fat or carbohydrate intolerance. In these situations a modular feeding approach should be used (see Table 7.24). There are three infant AAFs available in the UK: Alfamino, Neocate LCP and Nutramigen AA and Alfamino (Table 7.7). Neocate Spoon, which contains highly refined rice starch as a thickener, can be offered as a nutrient dense hypoallergenic weaning food if required.
Table 7.7 Infant formulas based on amino acids, per 100 mL
| Energy | |||||||||
| Dilution | CHO | Protein | Fat | Na | K | Osmolality | |||
| (%) | (kcal) | (kJ) | (g) | (g) | (g) | (mmol) | (mmol) | (mOsm/kg H2O) | |
| Neocate LCP | 13.8 | 67 | 279 | 7.2 | 1.8 | 3.4 | 1.1 | 1.8 | 340 |
| (SHS) | (4% MCT) | ||||||||
| Nutramigen AA | 13.6 | 68 | 286 | 7.0 | 1.9 | 3.6 | 1.4 | 1.9 | 348 |
| (Mead Johnson) | (3% MCT) | ||||||||
| Alfamino | 13.8 | 69 | 291 | 7.9 | 1.8 | 3.4 | 1.1 | 2.0 | 360 |
| (SMA) | (25% MCT) | ||||||||
First line treatment with, EHF or AAF
At present there is a paucity of evidence on which to base this decision in GI food allergy. The World Allergy Organisation (DRACMA) report reviewed the few randomised studies comparing AAF with EHF (both whey and casein based) in IgE mediated allergy and could not identify a net benefit of substituting cow’s milk with AAF over EHF. They recognise that the response to feeds in non-IgE mediated cow’s milk allergy may be different and plan to address this in future updates [38].The DRACMA guidelines have been summarised in Table 14.8. ESPGHAN continues to recommend EHF as first line treatment over AAF; however, AAF may be considered as first line therapy in infants with severe enteropathy indicated by hypoproteinaemia and faltering growth. The incidence of infants reacting to EHF is estimated to be <10%; however, this may be higher in infants with enteropathy or multiple food allergies [40]. One retrospective study found that up to 30% of food allergic infants, many with delayed reactions to foods, were intolerant of EHF [17]. Intolerances to hydrolysate formulas resulting in GI disturbances have been described [52].
There is a difference in cost between EHF and AAF with the latter being more than twice as expensive. The Health Improvement Network has shown that EHF was the most effective option in the treatment of cow’s milk allergy, using data from primary care organisations. There were no significant differences in clinical outcomes between the two types of feed [54]. Differences in prescribing patterns can be seen in a single geographical area which shows that current guidelines are not being followed [55].There is an urgent need for a prospective randomised controlled trial looking at the risk of intolerance of EHF in GI allergy.
Introduction of solids
Weaning should take place at the recommended age of around 6 months and not before 17 weeks. It is important to ensure that food offered is free of cow’s milk protein. Other dietary proteins that are most commonly implicated and may therefore need to be excluded include egg, soy and wheat. In very sensitive infants it may be wise to introduce new foods singly (p. 315).
Milk free diet
It is important that carers of infants requiring a cow’s milk protein free feed are given appropriate advice to enable them to exclude cow’s milk from solids. EU law (Directive 2003/89/EC, November 2005) regarding labelling of ingredients now means that products containing milk must be clearly identifiable. Exceptions include foods that are not prepacked such as bread from a bakery. Many manufacturers will now print an allergy statement on their packaging but, as this is not compulsory, parents should always be advised to check ingredients. Avoiding foods labelled with ‘may contain’ should be taken seriously in IgE mediated allergy but these foods do not generally need to be avoided by children with GI food allergy. Parents need clear advice about this due to the increasing number of foods carrying this label (p. 322). For foods manufactured outside the EU it is still necessary to teach families the following ingredients which indicate the presence of cow’s milk in a manufactured food: casein, hydrolysed caseinates, whey, hydrolysed whey, lactose, milk solids, non-fat milk solids, butter fat. Milk free dietary information is summarised in Table 7.8.
Table 7.8 Milk free diet
| Foods permitted | Foods to be excluded | Check ingredients |
| Milk substitute | All mammalian milks, cheese, yoghurt, fromage frais, ice cream, butter | Margarines |
| Vegetable oils | ||
| Custard made with milk | ||
| substitute, sorbet | ||
| Meat, fish, eggs, pulses | Sausages, pies, foods in batter or breadcrumbs | |
| Baked beans | ||
| All grains, dry pasta, flour | Pasta with cheese or milk sauce, milk bread, nan bread | Tinned pasta |
| Most breads, most breakfast cereals | ||
| Cream cakes, chocolate cakes | Bought cakes or biscuits | |
| Fruit and vegetables | Instant mashed potato | |
| Plain crisps, nuts | Flavoured crisps | |
| Sugar, jam, jelly | Milk chocolate, toffee | Plain chocolate |
| Marmite | Ketchup, salad dressings, soups | |
| Milk shake syrups and powder | Malted milk drinks | |
| Pop, juice, squash |
Exclusion of soy protein
In conditions where soy intolerance is present in addition to cow’s milk protein allergy, foods containing soy and milk protein should be excluded from the diet (Tables 7.8 and 7.9). Vegetable or soy oils and soya lecithin are normally tolerated by individuals sensitive to soy protein and should not need to be excluded from the diet except in severely affected individuals [56, 57].
Table 7.9 Foods containing soya protein
| All soy based products including tofu, tempeh and soy sauce |
| Texturised vegetable proteins |
| Breads, biscuits and cakes containing soy flour |
| Baby foods containing soy protein |
| Soy margarines |
Milk, egg, wheat and soy exclusion diets
In conditions where a simple exclusion diet has not worked or where there is a diet history suggestive of multiple food intolerances this dietary regimen may be tried. Soy, eggs and wheat are covered by EU law (Directive 2003/89/EC, November 2005) and must be clearly labelled on all prepacked foods. Families need a lot of help and information about commercial foods to enable them to adhere to this regimen. Suitable wheat free products that are available via the Advisory Committee on Borderline Substances (ACBS) cannot be prescribed for wheat allergy and a separate letter to the GP requesting help is often required. Many of these products will contain milk, egg or soya so families need help to identify suitable substitutes. For foods manufactured outside the EU it is still necessary to teach families the following ingredients which indicate the presence of egg, wheat and soya in manufactured foods: egg albumin, wheat flour, wheat starch/bran, edible starch, modified starch, hydrolysed wheat protein, rusk, batter, breadcrumbs, thickener (unless specified as being made from another cereal), soy protein isolate, soya grits, tofu, hydrolysed/spun/textured vegetable protein, miso, tempeh (Tables 7.10 and 14.6).
Table 7.10 Milk, egg, wheat and soya free diet
| Foods permitted | Foods to be excluded | Check ingredients |
| Milk substitute Vegetable oils | All mammalian milks and products, soya milks and soy products, shredded suet Eggs | Margarines |
| All meat, poultry, fish, shellfish (fresh or frozen), pulses | Meat or fish dishes with pastry, breadcrumbs or batter Tofu, tempeh, soy beans, Quorn | Sausages, beef and vegetarian burgers, hot dogs, ready meals |
| Rice, rice noodles or pasta, maize, corn pasta, cornflour, tapioca, sago, arrowroot, buckwheat, barley, oats, gram flour, potato flour, rye, ground almonds, carob, teff, amaranth, millet | Wheat, rye and soya flour, spelt flour, wheat bran or germ, semolina, couscous, tabouleh, pancakes, batter, pizza, stuffing mixes, ordinary pasta, e.g. spaghetti | Gluten free bread, cakes, biscuits, pasta Oatcakes |
| Breakfast cereals made from rice, corn and oats, rice and corn cakes | Wheat based breakfast cereals Bread, crispbreads, crackers, chapatti, croissants, biscuits, cake | Poppadoms |
| Jelly, plain custard powder, rice, tapioca or sago pudding (made with milk substitute) | Pies, pastries, mousse, trifle, cheesecake, instant desserts | Sorbet, blancmange powders |
| Fruit and vegetables | Potato croquettes | Vegetables in dressing, e.g. coleslaw, potato waffles, instant mashed potato |
| Plain crisps | Flavoured potato crisps | |
| Marmite, Bovril | Ordinary mayonnaise, salad creams, soy sauce | Stock cubes, gravy mixes, soups, sauces |
| Sugar, jam, honey, syrup, plain fruit lollies, milk shake syrup and powder, cocoa | Chocolate spread, lemon curd, milk chocolate, instant milk drinks | Plain chocolate, jelly sweets, marshmallows, baking powder, drinking chocolate, malted drinks |
Suitable feeds for older children
A suitable infant formula should be continued for as long as is nutritionally indicated in children on an exclusion diet and is preferable under the age of 2 years [38]. In situations where a large percentage of the child’s nutrition comes from a formula either it will need fortification to meet nutritional requirements or a feed designed for older children should be used. If an infant formula is modified care needs to be taken to ensure an appropriate protein to energy ratio. Rather than addition of energy supplements it is often preferable to concentrate the feed. This should be done slowly and taking into account individual tolerance [53]. Most feeds in Table 7.11 have been designed to meet the requirements of older children requiring hypoallergenic feeds. Adult feeds based on soy or hydrolysed protein should be used with care in older children and may require modification or vitamin and mineral supplementation. Not all the feeds are categorised as EHF and care needs to be taken when selecting a feed for cow’s milk allergy.
Table 7.11 Hydrolysate and amino acid based feeds for older children, per 100 mL
| Energy | |||||||||
| Dilution | CHO* | Protein | Fat† | Na | K | Osmolality | |||
| (%) | (kcal) | (kJ) | (g) | (g) | (g) | (mmol) | (mmol) | (mOsm/kg H2O) | |
| Hydrolysate feeds | |||||||||
| Pepdite 1+ | 23 | 100 | 420 | 13 | |||||