Gastric Dysplasia
Mikhail Lisovsky
Gregory Y. Lauwers
INTRODUCTION
Gastric epithelial dysplasia (GED), defined as histologically unequivocal neoplastic epithelium devoid of evidence of invasion into the lamina propria or submucosa, encompasses a wide spectrum of cytologic and/or architectural alterations. GED is recognized as precursor of gastric adenocarcinoma and also as a marker of risk for cancer elsewhere in the gastric mucosa.1 Evidence supporting that GED is a direct precursor of gastric adenocarcinoma stems primarily from observations in surgically resected gastric cancers. In this setting, high-grade dysplasia has been identified in 40% to 100% of early gastric cancers and 5% to 80% of advanced adenocarcinomas.2 Subsequent prospective and cohort studies have shown an increased risk of progression of dysplastic lesions to adenocarcinoma, which supports initial observations.3,4 Notably, while the more differentiated tubular (intestinal) type of gastric adenocarcinoma is associated with well-characterized dysplastic lesions, a specific precursor lesion for sporadic discohesive (diffuse) cancer is not as well defined (i.e., “neck dysplasia”) and its existence is questioned by some authors. As dysplastic lesions show molecular abnormalities similar to those of adenocarcinoma, the etiology of GED is thought to be the same as of gastric adenocarcinoma. Approximately 80% of gastric adenocarcinomas develop in the background of Helicobacter pylori atrophic gastritis; 5% are associated with Epstein-Barr virus; 5% arise in the setting of hereditary syndromes, such as Peutz-Jeghers, Cowden, Li-Fraumeni, hereditary nonpolyposis colonic cancer, hereditary diffuse gastric cancer, familial adenomatous polyposis (FAP), and juvenile polyposis.5, 6, 7 and 8 Rarer causes of gastric adenocarcinoma are autoimmune gastritis, atrophic gastritis in a gastric remnant, and Ménétrier disease.9,10 One of the consequences of this etiologic diversity is the heterogeneity of molecular pathways that leads to dysplasia and adenocarcinoma, which will be addressed later.
PREDYSPLASTIC MUCOSAL ABNORMALITIES (See Chapter By M. Rugge)
A widely accepted model of gastric carcinogenesis, where dysplasia plays an essential role has been described by Correa in the 1980s. Based on epidemiological and morphological studies, Correa postulated a sequence of progressive changes where chronic gastritis, mainly caused by H. pylori infection, causes mucosal atrophy, leading to intestinal metaplasia, then dysplasia and intestinaltype adenocarcinoma. Diffuse gastric cancer is thought not to arise through this cascade. The general validity of this pathway has been confirmed using mouse models.11 Gastric atrophy is defined as the loss of native glandular elements, with their replacement by stromal fibrosis and/or metaplasia of intestinal or pseudopyloric type. Currently, it is thought that metaplasia represents the first permanent alteration of the genetic/epigenetic program of precursor epithelial cells that give rise to metaplastic glands.12 Pseudopyloric metaplasia seen in the corpus/fundus shows a phenotype similar to antral or pyloric glands. It is characterized by expression of trefoil factor 2/spasmolytic polypeptide and by expression of pepsinogen I, a marker of chief cells. It is
also referred to as spasmolytic polypeptide-expressing metaplasia.13 In a seminal study, showing a role of pseudopyloric metaplasia in gastric carcinogenesis, stomachs with early intestinal-type adenocarcinoma were subjected to sectioning in toto. Atrophy was present as a continuous sheet of pseudopyloric metaplasia with islands of intestinal metaplasia seen only within the atrophic zone. All carcinomas were found within the atrophic zone, suggesting that the extent of mucosal atrophy defined by pseudopyloric metaplasia is more closely related to gastric carcinoma than is the presence or type of intestinal metaplasia.14 Independently, pseudopyloric metaplasia was found to be associated with >90% of gastric adenocarcinomas in patients from the United States, Japan, and Iceland, implicating it as an important preneoplastic lesion.15, 16 and 17 Furthermore, in a mouse model of gastric cancer caused by H. felis, pseudopyloric metaplasia appears to be an essential step and the only type of metaplasia observed.18,19 Finally, in transgenic mouse models expressing transcription factors Cdx1 and Cdx2, pseudopyloric metaplasia and gastric cancer developed consistently in Cdx2 transgenic mice but were not reported in the Cdx1 transgenic mice, although in both models, gastric mucosa converted to intestinal metaplasia.20,21
also referred to as spasmolytic polypeptide-expressing metaplasia.13 In a seminal study, showing a role of pseudopyloric metaplasia in gastric carcinogenesis, stomachs with early intestinal-type adenocarcinoma were subjected to sectioning in toto. Atrophy was present as a continuous sheet of pseudopyloric metaplasia with islands of intestinal metaplasia seen only within the atrophic zone. All carcinomas were found within the atrophic zone, suggesting that the extent of mucosal atrophy defined by pseudopyloric metaplasia is more closely related to gastric carcinoma than is the presence or type of intestinal metaplasia.14 Independently, pseudopyloric metaplasia was found to be associated with >90% of gastric adenocarcinomas in patients from the United States, Japan, and Iceland, implicating it as an important preneoplastic lesion.15, 16 and 17 Furthermore, in a mouse model of gastric cancer caused by H. felis, pseudopyloric metaplasia appears to be an essential step and the only type of metaplasia observed.18,19 Finally, in transgenic mouse models expressing transcription factors Cdx1 and Cdx2, pseudopyloric metaplasia and gastric cancer developed consistently in Cdx2 transgenic mice but were not reported in the Cdx1 transgenic mice, although in both models, gastric mucosa converted to intestinal metaplasia.20,21
CLINICAL FEATURES
The prevalence of GED is characterized by variation worldwide, from 9% to 20% in high-risk areas such as Colombia and China to 0.5% to 3.75% in Western countries where gastric cancer is less common.22,23 This difference is likely a result of variations in the genetic makeup of the respective populations, defined by polymorphisms of IL-1B, IL-1RN, and TNF-α and by environmental factors, including the prevalence of H. pylori infection. The genotype of H. pylori also affects the risk of neoplastic progression. For instance, cagA, vacA, and babA genotypes have been associated with increased cancer risk. Moreover, the frequency of GED varies with the underlying etiology. GED prevalence of up to 40% has been reported in patients with pernicious anemia.24 However, the disease confers an only moderately increased risk of gastric cancer.25 Patients with FAP are also at higher risk of developing either flat or, more commonly, polypoid dysplasia (i.e., adenomas). These adenomas typically are located in the antrum, are frequently multiple, and are observed in 2% to 5% of patients.26, 27, 28 and 29
Most patients diagnosed with GED are in the sixth to seventh decade of life,29,30 with men affected more often than women (male-female ratios range from 2.4 to 3.9:1).30,31 Although GED may be diagnosed in any segment of the stomach, it most commonly affects the lesser curvature, particularly the antrum or the incisura angularis.29,32 Although GED is often diagnosed on random biopsies without corresponding endoscopic abnormalities, various abnormal endoscopic patterns may be seen.30,31 These include mucosal irregularity in a background of atrophic mucosa, erosions, ulcers,30 mucosal scars,29 diffuse inflammatory changes,33 plaques, and polyps.29
HISTOLOGIC FEATURES, GRADING, AND CLASSIFICATION
GED is characterized by variable cellular and architectural atypia. In the United States, the term “adenoma” has been used when a discrete grossly appreciable lesion is observed or “dysplasia” when it is flat. However, in Japan, for example, the term “adenoma” encompasses all macroscopic types. GED is divided into several histologic subtypes and is graded according to the prominence of the atypia. The current two-tier scheme of low-grade and high-grade dysplasia has proven to be more reproducible than the obsolete three-tier system of mild, moderate, and severe dysplasia.34 Determination of the correct grade is critical, because it predicts both the risk of malignant transformation and the risk of developing gastric cancer elsewhere in the stomach. It also provides a clinically meaningful risk stratification method that can be used to guide patient management (see below).35
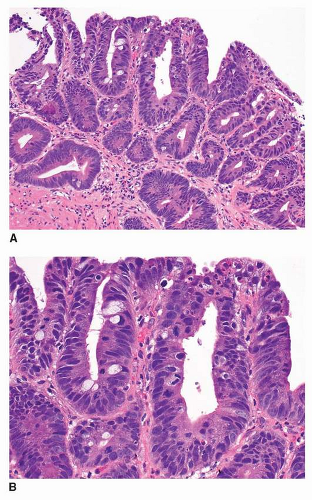
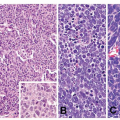
Most cases of GED have an intestinal phenotype resembling colonic adenomas and express intestinal type mucin Muc2 and CDX2.36,37 This type of dysplasia is referred to as adenomatous dysplasia or type I. When low-grade, it is characterized architecturally by crowded but simple tubular glands with little branching or budding. The glands are lined by atypical columnar cells with increased nuclear-to-cytoplasmic ratio and mucin depletion (Fig. 22-1A and B). The nuclei
are overlapping, pencillate, and hyperchromatic, with pseudostratification and inconspicuous nucleoli.38 The dysplastic cells extend to the mucosal surface, and this lack of surface maturation is an essential feature of dysplasia.34,35,39
are overlapping, pencillate, and hyperchromatic, with pseudostratification and inconspicuous nucleoli.38 The dysplastic cells extend to the mucosal surface, and this lack of surface maturation is an essential feature of dysplasia.34,35,39
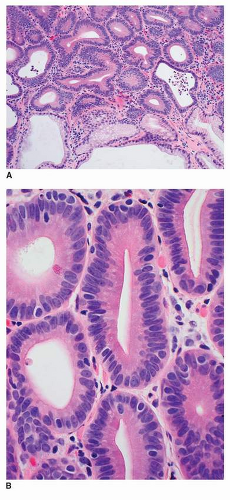 FIGURE 22-1 A,B: Low-grade adenomatous dysplasia, characterized by limited gland crowding, pencillate, hyperchromatic nuclei, and pseudostratification. |
High-grade dysplasia is characterized by marked architectural and cytologic abnormalities, and the diagnosis can be made when either abnormality is present unequivocally. Glands show back-to-back crowding and limited branching (Fig. 22-2A and B). Intraluminal necrotic debris is
commonly present. However, cribriforming and budding are not part of high-grade dysplasia and are consistent with intramucosal carcinoma. In contrast to low-grade dysplasia, dysplastic cells are usually cuboidal rather than columnar, with a high nuclear-cytoplasmic ratio. The nuclei vary in size, are round to oval, and are vesicular with prominent nucleoli and a thickened and irregular nuclear membrane. Cells show distinct loss of cell polarity, defined either as marked pseudostratification of the nuclei (to the level of the luminal surface) or as loss of the perpendicular orientation of the nuclei in relation to the basal membrane. Often, mitoses are numerous, and atypical mitoses also may be present.34,35,39
commonly present. However, cribriforming and budding are not part of high-grade dysplasia and are consistent with intramucosal carcinoma. In contrast to low-grade dysplasia, dysplastic cells are usually cuboidal rather than columnar, with a high nuclear-cytoplasmic ratio. The nuclei vary in size, are round to oval, and are vesicular with prominent nucleoli and a thickened and irregular nuclear membrane. Cells show distinct loss of cell polarity, defined either as marked pseudostratification of the nuclei (to the level of the luminal surface) or as loss of the perpendicular orientation of the nuclei in relation to the basal membrane. Often, mitoses are numerous, and atypical mitoses also may be present.34,35,39
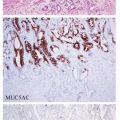
Other, less common, histologic variants of GED have been recognized as well. Foveolar-type dysplasia, also known as hyperplastic or type II dysplasia, is usually seen in nonintestinalized mucosa.38 It resembles normal surface-foveolar epithelium and lacks goblet and Paneth cells. The cells are cuboidal or low columnar and they variably have clear cytoplasm or retain eosinophilic apical mucin. Frequent immunoreactivity for the gastric-type mucin Muc5AC, but not for the intestinal mucin Muc2, is typical.36 Foveolar dysplasia has been noted in some series to be more commonly associated with poorly differentiated tubular adenocarcinoma.38,40 A significant proportion of gastric dysplasias, about 30%, was recently reported to have a hybrid adenomatous/foveolar phenotype, defined as having at least 10% of the alternative histologic type.36
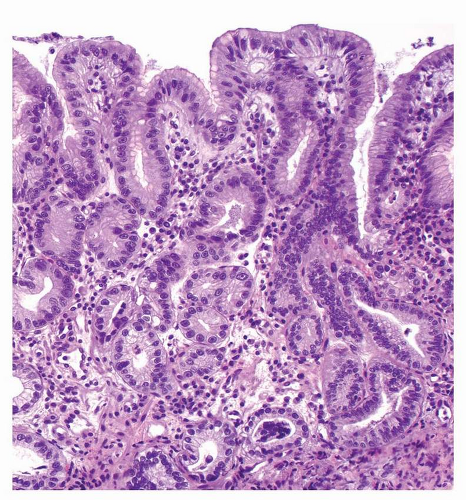 FIGURE 22-3 Low-grade foveolar dysplasia, with oval, basal nuclei, inconspicuous nucleoli, and mild stratification. |
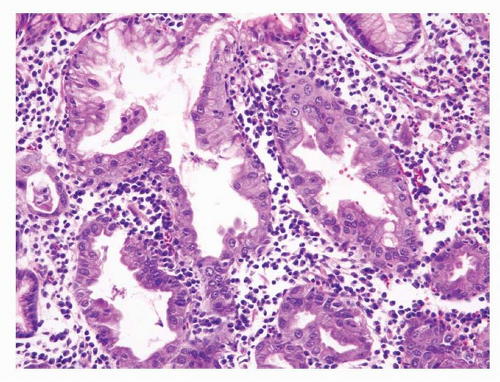
Stratification of foveolar GED into low- and high-grade categories is not well established, and the diagnosis of low-grade dysplasia can be challenging. The presence of architecturally simple gastric foveolar-type epithelium with elongated, hyperchromatic nuclei and some degree of pseudostratification is categorized as low-grade foveolar dysplasia (Fig. 22-3). High-grade foveolar dysplasia is characterized by complex glands of variable size and shape with papillary infoldings, serration, and occasional cystic dilatation. Nuclei are round to oval and vesicular with prominent nucleoli (Fig. 22-4).36
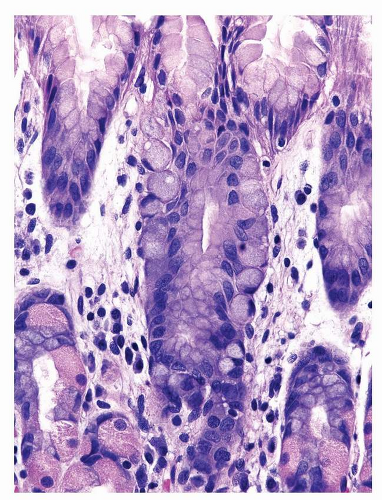
Finally, some dysplastic lesions show a pyloric phenotype characterized by cuboidal cells with eosinophilic cytoplasm and round nuclei and highlighted by Muc6 immunoreactivity (Fig. 22-5).41
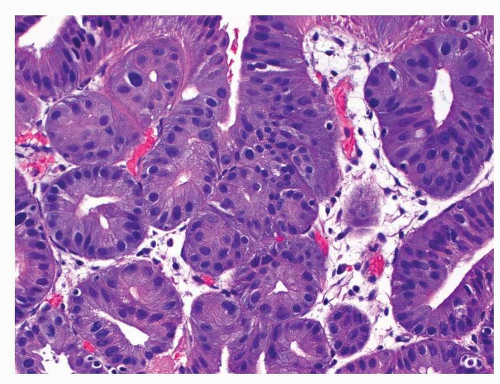 FIGURE 22-5 Low-grade pyloric-type dysplasia. The lesion is composed of small cuboidal cells with eosinophilic cytoplasm and round nuclei. |
Tubule neck dysplasia is a rare type of GED that is not well defined. It usually occurs in nonmetaplastic gastric epithelium and is thought to be a precursor of diffuse-type gastric carcinoma.42 Typical features include pale and polygonal or nondescript neoplastic cells confined to markedly elongated tubular structures occupying the midportion of the mucosa that corresponds to the mucous neck region.42 The cells of dysplastic tubules do not show evidence of mucous, parietal, or chief cell differentiation. Transition from dysplastic tubules to overt invasive diffuse cancer can be seen in deeper parts of the mucosa, and malignant cells of invasive cancer can infiltrate between dysplastic tubules43 (author’s unpublished observations). It remains to be shown whether tubule neck dysplasia can be (a) consistently diagnosed in the absence of admixed diffuse carcinoma and (b) be reliably differentiated from atypical reactive change.42
Another morphologic type of precursor lesions has been described in familial hereditary diffuse gastric cancer, an autosomal dominant cancer susceptibility syndrome, characterized by signet-ring cell (diffuse) gastric cancer and lobular breast cancer.44 It is caused by germ line E-cadherin/CDH1 gene mutations, and close to 50% of affected patients develop diffuse-type gastric cancer. In many prophylactic gastrectomies from these patients, examples of signet-ring cell carcinoma in situ have been reported. The latter corresponds to the presence of signet ring cells located within the confine of the basement membrane. From this location, the cells spread in pagetoid manner between the gastric epithelium and the basal membrane along the foveolar region45,46 (Fig. 22-6). These lesions have been found to be multifocal, and although one study showed their predilection for the distal stomach and the body-antral transitional zone, in another study, the majority of the lesions were found in the proximal third of the stomach.47,48 E-cadherin immunoreactivity is reduced or absent in these lesions.49
GED DEVELOPING IN BENIGN POLYPOID LESIONS
GED may develop in gastric hyperplastic polyps, particularly those >2 cm in size. Several studies have reported that dysplastic changes occur in 1.8% to 16.4% of hyperplastic polyps.50,51 In sporadic fundic gland polyps, dysplasia is exceedingly rare, but it is prevalent in patients with FAP, occurring in up to 42% of patients with FAP-associated fundic gland polyps.28,52 Gastric adenomas constitute approximately 7% to 10% of all gastric polyps53 and occur in 2% to 50% of patients with FAP26,33,54 Dysplasia can also occur in gastric juvenile polyps in patients with juvenile polyposis.55
DIFFERENTIATING DYSPLASIA FROM REACTIVE ATYPIA
The diagnosis of dysplasia can be challenging for multiple reasons, including orientation of the specimen, sampling, and difficulty in distinguishing it from reactive atypia. Also, while architectural and cytologic features are used in concert to reach a diagnosis, most criteria suffer from low specificity in isolation. In practice, these difficulties lead to significant interobserver diagnostic variability. Furthermore, mucosal regeneration caused by active inflammation or reactive/chemical gastropathy and reparative processes around erosions and ulcers may mimic dysplasia by demonstrating cytological atypia with severe mucin depletion, nuclear hyperchromasia, and increased mitotic activity. Clues to the reactive nature of the epithelial changes include the presence of vascular congestion and edema of lamina propria, evenly spaced nuclei without crowding or pseudostratification, gradual rather than abrupt transition between the atypical and adjacent normal cells, and maturation of the atypical cells of deeper glands as they reach the luminal surface (Fig. 22-7).34,35
Few markers are available to support a diagnosis of gastric dysplasia. p53 nuclear staining is reported in about one third of cases of dysplasias,56, 57,




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree