FUNCTIONS OF THE NONENDOCRINE HYPOTHALAMUS
TEMPERATURE REGULATION
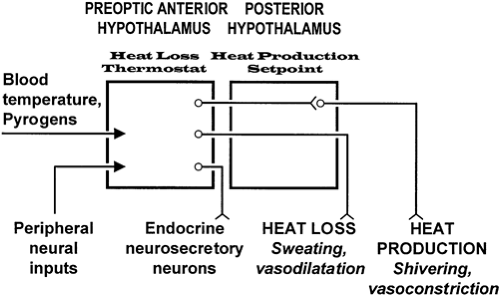
For the body to function efficiently, its temperature must be maintained within narrow limits. Wide variations beyond this range can result in serious metabolic derangement and death. The hypothalamus ensures that the heat gained by the body from metabolic activity, and in some circumstances from the environment, is balanced by the heat lost.21,22
The preoptic anterior hypothalamus contains thermosensitive neurons that monitor the temperature of blood (Fig. 9-3). Experiments have shown that serotonin (5-hydroxytryptamine) released in this area stimulates hypothalamic heat production centers. This effect is blocked by norepinephrine or epinephrine.
The caudolateral portion of the hypothalamus is insensitive to changes in body temperature, but the regulatory center that determines the normal setpoint of 37°C is located here. The injection of acetylcholine-like substances into this region causes profound and long-lasting hypothermia. It also is through this area that the main pathways controlling heat loss and heat conservation travel to the midbrain, pons, medulla, and spinal cord.
Intraventricular injection of the neuropeptides mammalian bombesin, neurotensin, TRH, somatostatin, and β-endorphin decreases body temperature and thus implicates these substances in thermoregulation. CRH appears to be an important mediator of thermogenesis in response to serotonin and its agonists and to cytokines. No peptide or group of peptides, however, has been singled out as the physiologic regulator of body temperature.20 In some experiments, prostaglandin E2 has increased body temperature.
Hypothalamic injury after head trauma or cerebral infarction can produce prolonged hypothermia resulting either from a change in the setpoint or from an impairment of heat production mechanisms. Paroxysmal hypothermia has been described in a few patients. These persons appear to have a temporary alteration in setpoint, with the body temperature falling to 32°C or lower. The hypothermia may last for minutes to days and is associated with fatigue, decreased alertness, hypoventilation, and even cardiac arrhythmia. The loss of body heat is caused by increased sweating and vasodilation. Although the paroxysmal nature of these episodes has suggested an epileptic etiology, the attacks are not prevented by use of anticonvulsants.21
Paroxysmal hyperthermia can occur in some conditions. Acute damage to the preoptic anterior hypothalamus from surgery, subarachnoid hemorrhage, or cerebral infarction can lead to profound impairment of heat loss mechanisms, and the resulting hyperthermia can be lethal. Cyclic hyperthermia has been seen in some patients, but the neuropathologic substrate for this condition is unknown. Some have responded to therapy with phenytoin. Sustained hyperthermia probably is not seen in hypothalamic dysfunction. Reported case studies of prolonged hyperthermia attributed to hypothalamic dysfunction have not excluded an underlying malignancy or unrecognized infection. Cyclic hypothermia, responsive to treatment with anticonvulsant agents, such as clonidine or cyproheptadine, has been described.23
Large lesions in the posterior hypothalamus or lesions in the brainstem that damage the hypothalamic outflow tracts may result in poikilothermia, a condition in which the body temperature varies with environmental temperature. Most affected patients have hypothermia, although in hot, humid conditions, hyperthermia may be a problem.21
During fever, the body’s temperature setpoint is elevated, although the ability to regulate temperature around the new setpoint is normal.24 In response to an infection or other cause of inflammation, the body’s inflammatory cells—primarily monocytes—release cytokines,25 which act at the hypothalamus to cause fever.24 Interleukin-1 (IL-1) releases phospholipases in the hypothalamus that, in turn, release arachidonic acid from plasma membranes. Arachidonic acid causes a rise in prostaglandin E, which raises the body temperature setpoint. Treatment with acetylsalicylic acid or acetaminophen to reduce fever probably affects this process. Animal studies suggest that the action of IL-1 occurs in the preoptic anterior hypothalamus through the reduction of the sensitivity of “warm-sensitive” neurons, allowing the body to tolerate a higher temperature. Once this new setpoint has been established, the hypothalamus uses normal physiologic mechanisms to maintain body temperature by peripheral vasoconstriction, reduced sweating, and, if necessary, increases in heat production through shivering.
Tumor necrosis factor (TNF) is another cytokine that alters the setpoint in the hypothalamus and increases the production of IL-1 locally, in the hypothalamus. Most TNF seems to be produced in macrophages stimulated by bacterial endotoxin. Inter-leukin-6 (IL-6) and interferon-γ also act directly at the hypothalamus to raise the setpoint.
The exact role of the cytokines in regulating body temperature and in causing fever is unclear because complex interactions exist among these compounds. IL-1, for example, stimulates its own production and interferon-γ stimulates IL-1 production, whereas interleukin-4 suppresses the production of IL-1, TNF, and IL-6. IL-1 production also is inhibited by glucocorticoids and prostaglandin E.
The fulminant hyperthermia (malignant hyperthermia) that can occur during anesthesia is not hypothalamic in origin. Rather it results from excessive muscle contraction caused by an abnormality of the muscle membrane. Another syndrome of hyperthermia is the neuroleptic malignant syndrome. This condition, characterized clinically by hyperthermia, rigidity of skeletal muscles, autonomic instability, and fluctuating levels of consciousness, has been associated with the use of major tranquilizers, rapid withdrawal from treatment with dopaminergic agents (e.g., L-dopa or bromocriptine), and, less commonly, the use of tricyclic antidepressants. The common denominator in the syndrome seems to be an alteration in dopamine function in the hypothalamus. Treatment consists of discontinuing the use of neuroleptics, providing general support, and administering anticholinergics for mild cases, bromocriptine (5 mg orally or nasogastrically four times daily) for more severe cases, and benzodiazepines or dantrolene (2–3 mg/kg per day intravenously to a maximum of 10 mg/kg per day) for resistant cases.26,27
APPETITE REGULATION
In most animals, the body is able to balance its intake of food and output of energy to maintain body weight. It is the hypothalamus that receives inputs from the periphery that either stimulate or inhibit the intake of food, and it is the hypothalamus that, likewise, sends signals to other parts of the brain to influence endocrine, autonomic, and motor nervous system function.28 The interaction between the limbic system and the hypothalamus is critical in translating the need for food into behaviors such as hunting and stalking.
The destruction of both ventromedial nuclei in the rat or the cat markedly increases food intake for a few days. As the animal becomes obese, the overeating decreases; if it is then fasted back to ideal weight and given free access to food, the animal again increases its food intake until it becomes obese. Lesions of the ventromedial nuclei, however, do not produce a pure syndrome of obesity. During the phase of overeating, the animals often are irritable and aggressive, becoming lethargic and passive when the increased weight is achieved. The pituitary gland is not necessary for the weight gain because hypophysectomized animals also become obese. If hyperphagia is prevented by tube feeding, the animals still become obese. Hyperinsulinemia has been observed in lesioned animals within minutes of surgery; if this is prevented by lesioning the pancreatic B cells with streptozocin, the obesity and hyperphagia are prevented.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree