Summary of Key Points
- •
The determination of tumor histology in nonsmall cell lung cancer (NSCLC) has become essential in treatment decision-making due to differential efficacy and toxicities seen with newer therapies.
- •
International Association for the Study of Lung Cancer (IASLC) guidelines recommend testing all patients with lung adenocarcinoma for both EGFR mutations and ALK rearrangements.
- •
Further molecular profiling of both adenocarcinoma and squamous cell lung cancers has identified novel driver mutations that are being investigated as potential targets of new therapies.
- •
Platinum doublet chemotherapy is the established standard first-line therapy in patients with advanced or metastatic NSCLC.
- •
The duration of platinum-based first-line therapy should be four to six cycles.
- •
Triple drug chemotherapy in NSCLC does not improve survival and often results in increased toxicity.
- •
ERCC1 and RRM1 have not been useful as predictive biomarkers for selection of chemotherapy based on prospective randomized clinical trials.
- •
Bevacizumab has been approved in combination with chemotherapy for the first-line therapy of patients with advanced NSCLC with nonsquamous histologies. Other antiangiogenic therapies in NSCLC have been disappointing.
- •
Patients with activating mutations in EGFR benefit from upfront therapy with EGFR tyrosine kinase inhibitors.
- •
Patients with ALK rearrangements can be successfully treated with ALK inhibitors, such as crizotinib.
- •
For patients with tumor PDL-1 expression >50%, pembrolizumab, an immune checkpoint inhibitor, is superior to platinum-based chemotherapy.
- •
Elderly patients with advanced NSCLC benefit from combination chemotherapy; selection of appropriate patients is vital.
- •
Patients with borderline performance status can also benefit from combination chemotherapy; patient selection requires careful consideration of comorbidities.
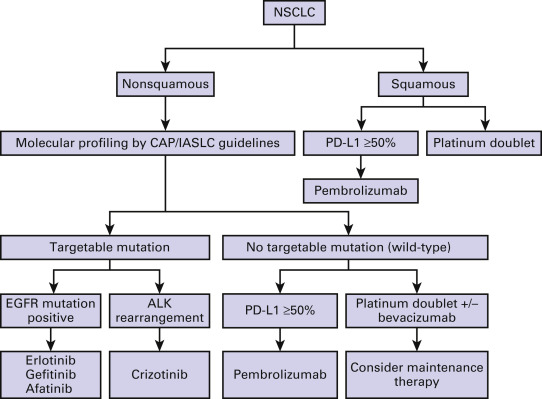
Lung cancer presents at an advanced stage at the time of diagnosis in the majority of patients. The overall goals of treatment for advanced stage disease are palliation and improvement in survival. Local treatment modalities such as radiotherapy and surgery play a limited role and are implemented primarily for symptom control. Systemic therapy remains the principal therapeutic modality for advanced stage nonsmall cell lung cancer (NSCLC). Until the late 1990s, treatment of advanced lung cancer followed the straightforward algorithm of platinum-based combination therapy, irrespective of histologic subtype, without any option for further lines of treatment. With the introduction of so-called third-generation cytotoxic drugs, the treatment of NSCLC changed and overall survival improved to approximately 8 months for patients with a good performance status. In the past two decades, there has been a gradual shift in therapy from the use of systemic chemotherapy in all patients, to the current approach in which histology and molecular status play a key role in treatment selection ( Fig. 44.1 ). This has been made possible by greater insights into lung cancer biology, the availability of novel therapeutic agents, and the increasing focus on identification of biomarkers to guide therapy. As a result, while a cure from advanced NSCLC still remains elusive, a significant subset of patients has long-term survival and an improved quality of life.
Prognostic Factors in Nonsmall Cell Lung Cancer
The assessment of prognosis is an important factor affecting the selection of appropriate treatment for each individual patient. The variables that are associated with prognosis can be grouped into categories: tumor-related, such as primary site, histology, and extent of disease; patient-related, such as performance status, comorbidity, and sex; and environmental factors, such as nutrition and the choice and quality of treatment.
Clinical Factors
Performance status and comorbid conditions are amongst the most important prognostic factors. Moreover, these determinants are also of utmost importance for the selection of therapy, as outlined later. The systematic determination of comorbidities is an essential component to preselect appropriate chemotherapy regimens and to provide the best supportive care.
In addition to noncancer-related comorbidities, patients also suffer from symptoms related to the primary tumor, mediastinal spread, or paraneoplastic syndromes. Moreover, lung cancer commonly produces systemic effects such as anorexia, weight loss, weakness, and profound fatigue. In a study of 12,428 NSCLC patients in the international staging database of the International Association for the Study of Lung Cancer (IASLC), performance status, age, and gender appeared to be independent prognostic factors for survival in addition to clinical stage. In advanced NSCLC, some routine laboratory tests (mainly white blood cells and hypercalcemia) were also found to be significant prognostic variables. Nowadays, the clear majority of lung cancer cases are diagnosed in patients aged >65 years. Age at diagnosis is another important factor that needs to be considered for therapy decision making. Often, increasing age is accompanied by multiple comorbidities, which further limit therapeutic options and outcome of the patient.
Ethnicity
While lung cancer remains a leading cause of mortality for all races, recent research has focused on ethnic variations in this disease. One of the most striking disparities seen is the difference in lung cancer risk and survival for African and Asian ethnicities. For example, the epidermal growth factor receptor ( EGFR ) mutation rate differs considerably between various ethnicities. Epidemiologic research has focused on behavioral, cultural, and socioeconomic factors that may influence risk, although no clear link has been established. Access to care is also variable among various ethnic groups and remains an important barrier to the delivery of optimal therapy.
Tumor Stage
The anatomic extent of the disease, as described by the TNM classification, is the most important prognostic factor in NSCLC. The seventh edition of the TNM classification that came into effect in 2010 derived from the analysis of the largest database ever generated for this purpose, with data from 46 sources in more than 19 countries around the world and with information about patients treated with all modalities of care. An important change involved the recognition that patients with extrathoracic disease have a slightly less favorable outcome compared with patients with metastatic spread confined to the thorax, even within the stage IV category. This has resulted in the division of stage IV to M1a and M1b based on the presence or absence of extrathoracic metastasis. It has also been recognized that malignant pleural or pericardial effusions portend a particularly poor prognosis among those with stage IV disease. In recognition of the importance of this, malignant effusions were moved from stage IIIB to IV disease in the seventh edition.
With additional cases analyzed in this international database, there will be further changes to the TNM classification system forthcoming in the eighth edition. The increased importance of T stage in prognosis has resulted in upstaging of tumors greater than 5 cm to T3 and those greater than 7 cm to T4. There will also be a new staging grouping, IIIC, created for patients with N3 nodal involvement and T3 or T4 primary tumors, to reflect the worse prognosis of these most locally advanced tumors. Finally, within metastatic disease, patients with a solitary metastasis in a single extrathoracic organ will be classified as M1b. Presence of oligometastases merits consideration of local therapies in addition to systemic therapy. These patients have similar survival to patients classified as M1a with lung, pleural or pericardial involvement and will share the stage IVA designation. The majority of metastatic patients who present with multiple metastatic lesions will be considered M1c disease and will be characterized as stage IVB.
Histology
The distinction between squamous and nonsquamous histology was the first step in the personalized treatment of patients with advanced NSCLC. Hence, accurate diagnosis of tumor histology has become essential in treatment decision-making and can impact considerations of both toxicity and potential efficacy of selected agents used in the management of this disease. For example, the use of the anti–vascular endothelial growth factor (VEGF) antibody bevacizumab is associated with a higher risk of pulmonary bleeding when used in patients with predominantly squamous cell histology. Also, the cytotoxic drug pemetrexed was found to be inactive in patients with squamous NSCLC. Therefore, the classification of NSCLC into the major categories of squamous cell carcinoma, adenocarcinoma, and large cell carcinoma is critical for treatment decisions. However, histologic subclassification of NSCLC remains a challenge for many reasons. The tumor is very heterogeneous in every aspect: pathology, presence of molecular alterations, radiographic appearance, clinical presentation, and response to systemic therapy. The initial diagnostic biopsies often have a limited amount of material that is inadequate to conduct necessary tests to identify histology and genotype. The availability of immunostains such as TTF-1, p63, and p40 has greatly improved the accuracy of histologic subclassification.
Molecular Markers
NSCLC tumors often harbor mutations in a number of critical genes such as p53, K-RAS, and LKB-1. The prognostic relevance of these mutations continues to be defined for patients with advanced NSCLC. Certain markers have gained attention because they also harbor predictive value. For example, the presence of an activating EGFR mutation translates into both predictive and prognostic information. Patients with EGFR mutation have overall better outcomes compared with those with wild-type EGFR , and also derive robust benefits from specific therapeutic inhibitors of the EGFR pathway. Similarly, limited early evidence indicates that patients with abnormal anaplastic lymphoma kinase ( ALK ) gene rearrangement have higher risk of recurrence following surgery for early-stage disease and a higher clinical benefit with pemetrexed therapy. The prognostic role of K-Ras mutations in lung adenocarcinoma has been debated extensively. Earlier evidence suggested poor sensitivity to chemotherapy and overall prognosis with K-Ras mutation, but emerging recent data have failed to confirm this. K-Ras mutated patients appear to have a very low likelihood of objective response with EGFR inhibitors. The knowledge of the prognostic and predictive potential of various molecular markers is bound to increase significantly in the coming years as molecular testing is adopted to routine practice settings.
Treatment of Advanced Nonsmall Cell Lung Cancer
Systemic Chemotherapy
Systemic chemotherapy prolongs survival and leads to symptom palliation compared with best supportive care alone for patients with advanced NSCLC. Similar to therapeutic developments for other solid tumors, the efficacy of a variety of cytotoxic agents has been tested in both preclinical and clinical studies in NSCLC in the last decades. Initial results on single-agent therapy, including cisplatin (CDDP), ifosfamide, vinblastine, vindesine, etoposide, and mitomycin-C, indicated limited activity leading to objective response rates of ≤15% and median response durations of 2 months to 3 months. However, complete responses after these treatments were rare, and their benefit on median survival, with the exception of cisplatin, was inconsistent. The relatively modest efficacy and the considerable toxicity of these cytotoxic agents led to considerable nihilism regarding the use of chemotherapy for NSCLC for many years. Starting in the mid-1980s, several novel cytotoxic drugs were evaluated in NSCLC, such as vinorelbine, paclitaxel, docetaxel, irinotecan, gemcitabine, and oxaliplatin, which showed response rates of 20% to 25% ( Table 44.1 ).
| Cytotoxic Agent | Patients ( n ) | Response Rate (%) | Median Survival (months) | Reference (year) |
|---|---|---|---|---|
| Vinorelbine | 206 | 14 | 7.2 | Le Chevalier (1994) |
| Irinotecan | 129 | 21 | 10.6 | Negoro (2003) |
| Cisplatin | 206 | 17 | 8.1 | Gatzemeier (2000) |
| Cisplatin | 262 | 11 | 7.6 | Sandler (2000) |
| Cisplatin | 219 | 14 | 6.4 | Von Pawel (2000) |
| Cisplatin | 209 | 12 | 6 | Wozniak (1998) |
| Gemcitabine | 84 | 20 | 6.7 | Vansteenkiste (2001) |
| Gemcitabine | 170 | 12 | 9 | Sederholm (2002) |
| Docetaxel | 152 | 22 | 8 | Georgouilas (2004) |
| Paclitaxel | 277 | 17 | 6.7 | Lilenbaum (2005) |
Combinations of various agents have also been evaluated in patients with NSCLC. Two meta-analyses showed a clear significant survival advantage for a two-drug regimen versus monotherapy, but on the other hand also demonstrated a significant increase in hematologic and nonhematologic side effects. Among several combinations, platinum-based chemotherapy was shown to lead to higher response rates and prolonged survival in comparison with monotherapy, albeit with the cost of increased toxicity. Given the limited availability of supportive care for chemotherapy-related toxicities in the early 1990s, the use of chemotherapy in patients with metastatic NSCLC was still under debate despite the consistent evidence for its modest activity.
Platinum Compounds
Platinum compounds form DNA adducts, which ultimately result in activation of p53-dependent and p53-independent apoptosis. As monotherapy, cisplatin has anticancer activity comparable with that of other single agents, leading to response rates of approximately 15% and a median survival of 6 to 8 months. In order to increase the efficacy of systemic treatment, several combination regimens have been extensively studied ( Table 44.2 ). Several randomized trials as well as meta-analyses provided scientific evidence that platinum-based combination therapy prolonged the survival of patients with advanced NSCLC. In 1995, a meta-analysis using updated data on 1190 patients with advanced NSCLC from 11 randomized clinical trials was published. The results, updated in 2008, demonstrated a 27% reduction in the risk of death for patients treated with cisplatin-containing regimens compared with supportive care alone, which translated to an absolute improvement in survival of 10% (5% to 15%) at one year.
| Regimen | Patients | Response Rate (%) | Median Survival | p | Reference (year) |
|---|---|---|---|---|---|
| Cisplatin/vindesine | 200 | 19 | 7.4 | 0.04 | Le Chevalier |
| Cisplatin/vinorelbine | 206 | 30 | 9.2 | (1994) | |
| Cisplatin/vinorelbine | 202 | 28 | 8 | NS | Kelly (2001) |
| Carboplatin/paclitaxel | 206 | 25 | 8 | ||
| Carboplatin/paclitaxel | 201 | 32 | 9.9 | NS | Scagliotti |
| Cisplatin/vinorelbine | 201 | 30 | 9.5 | (2002) | |
| Cisplatin/gemcitabine | 205 | 30 | 9.8 | ||
| Cisplatin/paclitaxel | 305 | 21 | 7.8 | NS | Schiller (2002) |
| Cisplatin/gemcitabine | 288 | 22 | 8.1 | ||
| Cisplatin/docetaxel | 289 | 17 | 7.4 | ||
| Carboplatin/paclitaxel | 290 | 17 | 8.1 | ||
| Cisplatin/vinorelbine | 404 | 25 | 10.1 | Fossella (2003) | |
| Cisplatin/docetaxel | 408 | 32 | 11.3 | 0.04 a | |
| Carboplatin/docetaxel | 406 | 24 | 9.4 | NS a | |
| Cisplatin/vindesine | 151 | 21 | 9.6 | 0.01 | Kubota (2004) |
| Cisplatin/docetaxel | 151 | 37 | 11.3 | ||
| Cisplatin/vindesine | 122 | 32 | 10.9 | 0.12 | Negoro (2003) |
| Cisplatin/irinotecan | 129 | 44 | 11.5 |
Compared with older regimens such as cisplatin with vindesine or vinblastine, cisplatin and mitomycin-C with vinblastine or vindesine, or cisplatin with etoposide, combinations of cisplatin with newer drugs (referred to as third-generation drugs: gemcitabine, taxanes, vinorelbine, topoisomerase I inhibitors) seem to exert somewhat higher efficacy and improved tolerability. For example, compared with platinum–gemcitabine combinations, several studies indicated inferior response rates, time to progression, and median overall survival (OS) for cisplatin, ifosfamide and mitomycin, cisplatin and vindesine, or cisplatin and etoposide regimens. Hematologic toxicity, especially thrombocytopenia, was pronounced in the gemcitabine-platinum groups whereas nonhematologic side effects appeared more frequently in the “classic” arms. Moreover, Le Chevalier et al. showed a significantly better response rate and survival for cisplatin–vinorelbine compared with cisplatin–vindesine.
The choice of the newer agent (gemcitabine, paclitaxel, or vinorelbine) that is combined with cisplatin does not seem to significantly affect the treatment efficacy (see Table 44.2 ). For instance, phase III studies (e.g., Southwest Oncology Group [SWOG] 9509) failed to demonstrate superiority of carboplatin–paclitaxel over cisplatin–vinorelbine in 408 patients. Similarly, the Italian Lung Cancer Study Group failed to detect any significant difference in outcome for cisplatin–gemcitabine, carboplatin–paclitaxel, and cisplatin–vinorelbine in 612 patients with previously untreated advanced NSCLC. However, both studies demonstrated differences between these regimens regarding their toxicity profiles. In the largest study that included 1207 patients (Eastern Cooperative Oncology Group [ECOG] 1594), Schiller et al. found no significant efficacy differences among the regimens cisplatin–paclitaxel, cisplatin–gemcitabine, cisplatin-docetaxel, and carboplatin–paclitaxel regarding response rates (17% to 22%) and median survival (7.4–8.1 months). Differences were only noted in toxicity profiles, with cisplatin–gemcitabine causing more thrombocytopenia, cisplatin–docetaxel causing more neutropenia, and the carboplatin–paclitaxel arm experiencing the lowest rate of potentially life-threatening toxicities. Another phase III study (TAX 326) randomized 1218 patients to receive cisplatin–docetaxel, carboplatin–docetaxel, or the control arm of cisplatin–vinorelbine. Patients treated with cisplatin–docetaxel had a higher response rate (31.6% vs. 24.5%, p = 0.029) and median survival (11.3 vs. 10.1 months, p = 0.044). Based on these observations, platinum-based chemotherapy remains the standard therapy in advanced or metastatic NSCLC. With the current combination partners, a plateau of efficacy has been reached.
Cisplatin Versus Carboplatin
Carboplatin is another platinum derivate with a tenfold longer half-life than cisplatin. Due to structural differences from cisplatin, it exhibits lower reactivity and slower DNA binding kinetics in vitro. In clinical studies, the nonhematologic tolerability of carboplatin is superior to that of cisplatin, making it a more convenient platinum analog for palliative chemotherapy.
Several studies have compared the efficacy of cisplatin with carboplatin in the management of advanced NSCLC. Rosell et al. reported a significantly improved survival for cisplatin–paclitaxel compared with carboplatin–paclitaxel with a higher rate of nonhematologic side effects in the cisplatin arm and a higher rate of neutropenia and thrombocytopenia in the carboplatin arm. In the TAX 326 trial, there was a nonsignificant trend toward improved survival for the combination of cisplatin–docetaxel over carboplatin–docetaxel. In contrast, the ECOG 1594 trial noted similar survival duration between the cisplatin and carboplatin-based treatment arms. However, there was a lower incidence of nonhematologic events such as nausea, vomiting, nephrotoxicity, and neurotoxicity with carboplatin-based therapy. This observation has also been made in other smaller trials with carboplatin-based regimens.
A meta-analysis using data from eight trials demonstrated that cisplatin-based chemotherapy offered a significantly higher objective response rate compared with carboplatin-based chemotherapy (odds ratio [OR], 1.36; 95% confidence interval [CI], 1.15 to 1.61; p = 0.001) and a nonsignificant improvement in survival (hazard ratio [HR], 1.050; 95% CI, 0.907 to 1.216; p = 0.515). In this meta-analysis, a subgroup analysis of the five trials that incorporated cisplatin or carboplatin with a new agent identified a significantly superior median survival for cisplatin-treated patients (HR, 1.106; 95% CI, 1.005–1.218; p = 0.039). These data were confirmed by another meta-analysis including 2968 patients from nine trials. Cisplatin-treated patients experienced a significantly higher response rate (OR, 1.37; 95% CI, 1.16–1.61; p < 0.001). Moreover, cisplatin-based treatment was associated with an improved median OS relative to treatment with carboplatin (9.1 versus 8.4 months; HR, 1.07; 95% CI, 0.99–1.15; p = 0.1) that was significant in subgroup analyses for patients with nonsquamous tumors (HR, 1.12; 95% CI, 1.01–1.23) and those treated with third-generation chemotherapy (HR, 1.11; 95% CI, 1.01–1.21). However, cisplatin-based chemotherapy was associated with more severe nausea, vomiting, and nephrotoxicity while severe thrombocytopenia was more frequent during carboplatin-based chemotherapy. Hence, the selection of the platinum compound should be made based on the regimen most likely to result in the best therapeutic index. In recent years, the availability of effective antiemetic agents has improved the therapeutic index of cisplatin-based regimens.
Triplets for Advanced NSCLC
To further increase the efficacy of systemic therapy in advanced NSCLC, a series of studies have evaluated the potential role for the use of three-drug regimens. These studies have consistently demonstrated that three-drug regimens are associated with higher toxicity, have at times higher objective response rates, but offer no statistically significant improvement in survival as compared with that offered by standard doublets ( Table 44.3 ). For example, a phase III trial of 557 stage IIIB/IV NSCLC randomized patients to receive cisplatin–gemcitabine for six cycles, cisplatin–gemcitabine–vinorelbine for six cycles, or three cycles of gemcitabine–vinorelbine followed by three cycles of vinorelbine–ifosfamide. Response rates were inferior for the nonplatinum sequential doublet while no differences in median survival or time to progression were observed. Predictably, toxicity was significantly higher for the triplet regimen. Similarly, a recent phase II study found no statistically significant difference between doublet (cisplatin–gemcitabine or gemcitabine–vinorelbine) and triplet (cisplatin–ifosfamide–gemcitabine, or gemcitabine–ifosfamide–vinorelbine) combinations; however, grade 3–4 leukopenia was significantly more common in triplets.
| Doublet Chemotherapy | Triplet Chemotherapy | Response Rate (%) | Grade 3–4 Neutropenia | Grade 3–4 Thrombocytopenia | Grade 3–4 Nausea/Vomiting |
|---|---|---|---|---|---|
| Cisplatin/gemcitabine | Cisplatin/gemcitabine/ifosfamide | 42 vs. 41 | 32 vs. 57 | 4 vs. 19 | 22 vs. 32 |
| Cisplatin/gemcitabine or gemcitabine/vinorelbine | Cisplatin/ifosfamide/gemcitabine or gemcitabine/ifosfamide/vinorelbine | 29 vs. 28 | 36 vs. 44 | 16 vs. 20 | 8 vs. 7 |
In a systematic overview, third generation triplet therapy had a significantly higher response rate (OR, 1.33; 95% CI, 1.50–2.23; p < 0.001) compared with standard doublet therapy; however, median survival (MR, 1.10; 95% CI, 0.91–1.35; p = 0.059) was not statistically different and the incidence of grades 3–4 hematologic toxicity, neuropathy, and diarrhea was significantly increased with triplet therapy. Based on these results, platinum-based doublet chemotherapy remains the standard first-line treatment for patients with metastatic NSCLC.
Platinum-Free Versus Platinum-Based Chemotherapy
In daily practice, several types of patients with advanced NSCLC are not optimal candidates to receive platinum-based chemotherapy due to the presence of certain comorbid conditions such as renal insufficiency, borderline performance status (PS), or preexisting sensory neuropathy. Hence, studies were conducted to evaluate whether the combination of two newer chemotherapeutic agents may be better suited for first-line therapy. In some earlier studies, a trend toward a higher survival was observed in patients treated with platinum-based combinations compared with those treated with platinum-free regimens. In a recent phase II study, a total of 433 stage IIIB–IV NSCLC patients received cisplatin–gemcitabine, gemcitabine–vinorelbine, cisplatin–ifosfamide–gemcitabine, or gemcitabine–ifosfamide–vinorelbine. Platinum-based regimens had a significantly longer overall survival (11.3 vs. 9.7; p = 0.044) compared with the other treatment arms but also resulted in higher incidence of grade 3–4 toxicity. In a meta-analysis based on abstracted data from randomized phase II and III studies, D’Addario et al. observed a significantly higher 1-year survival rate for platinum-based combinations compared with the nonplatinum regimens (34% vs. 29%; OR, 1.21; 95% CI, 1.09–1.35; p = 0.0003). However, when single-agent trials were excluded and platinum-based therapies were compared with third-generation–based combination regimens only, no statistically significant difference could be found (1-year survival, 36% for platinum regimens vs. 35% for nonplatinum regimens). In a more recent systematic review of randomized controlled trials comprising 4920 patients, the use of cisplatin-based doublet regimens was associated with a higher 1-year-survival rate (Hazard Ratio (HR), 1.16, 95% CI, 1.06–1.27; p = 0.001) compared with nonplatinum regimens, but also with an increased risk of anemia, neutropenia, neurotoxicity, and nausea. Conversely, carboplatin-based doublet regimens were associated with a similar 1-year survival rate (HR = 0.95, 95% CI, 0.85–1.07; p = 0.43) to that of nonplatinum doublets. Taken together, nonplatinum regimens do not have a clearly defined role in NSCLC and are only considered appropriate for patients that are not candidates for platinum agents.
Duration of Chemotherapy
The commonly used NSCLC chemotherapy regimens are administered in 3- or 4-week cycles. Imaging studies are recommended every two to three cycles to assess response to therapy. For patients that achieve an objective response or stable disease, the number of cycles of therapy has been a subject of several randomized studies. Socinski et al. randomized advanced NSCLC patients to treatment with the combination of carboplatin and paclitaxel for either four cycles or to continuation of therapy until disease progression. Interestingly, the median number of treatment cycles administered in both arms was four. There was no statistically significant improvement in OS for the extended chemotherapy approach. Predictably, toxicity was more common with administration of chemotherapy beyond four cycles. Another study by Smith et al. that compared three cycles of chemotherapy with six cycles also found no improvement in OS with the latter. In a systematic meta-analysis including 13 randomized control trials, 3027 patients receiving first-line (largely platinum-based) chemotherapy for three to four cycles were compared with patients with continuation of the same chemotherapy for six cycles or until disease progression. While extending chemotherapy substantially improved progression-free survival (PFS; HR, 0.75; 95% CI, 0.69–0.81; p < 0.00001), there was a statistically significant, but clinically only modest reduction in the hazard for death as compared with standard duration of chemotherapy over three to four cycles (HR, 0.92; 95% CI, 0.85–0.99; p = 0.03). Moreover, extension of chemotherapy was associated with higher toxicity and impaired quality of life. These findings were confirmed in a recent systematic review and meta-analysis of randomized trials comparing six versus fewer cycles of only platinum-based chemotherapy that had individual patient data available for analysis. While an improvement in PFS was observed in the four eligible studies with 1139 patients (HR 0.79; 95% CI, 0.68–0.90; p = 0.0007), there was no overall survival benefit to receipt of six cycles of platinum-based chemotherapy (median 9.54 months versus 8.68 months with fewer cycles; HR 0.94; 95% CI, 0.83–1.07; p = 0.33). This was independent of histology, sex, performance status, and age. Hence, most guidelines recommend limiting the duration of platinum-based first-line therapy to four to six cycles and prefer considering the induction of maintenance therapy.
Maintenance Therapy
After four to six cycles of first-line or induction chemotherapy, approximately two-thirds of patients have nonprogressive disease. Continuation of first-line platinum-based combination regimens beyond four to six cycles results in heightened toxicities and diminished quality of life without providing a major survival advantage. Thus, the standard therapeutic approach has entailed stopping treatment at that point, close clinical and radiographic surveillance, and initiation of second-line treatment at the time of progression. This “wait and watch” approach is frequently chosen after achieving maximal response to initial therapy. However, a “drug holiday” is often associated with patient anxiety about disease recurrence or progression coupled with concerns for clinical deterioration and the inability to receive subsequent therapy.
The availability of effective newer cytotoxic and molecularly targeted agents with overall good tolerability and low toxicity profile has led to the concept of maintenance therapy in order to maintain or improve the disease burden after completion of first-line therapy. Maintenance therapy involves either switching to a different compound (switch maintenance) or continuation of one drug partner of the induction regimen (continuation maintenance) in patients with a response or at least stabilization of disease. The role of maintenance therapy is discussed extensively in Chapter 46 .
Importance of Histology in the Treatment of NSCLC
NSCLC includes many histologic subtypes including adenocarcinoma, squamous cell carcinoma, and large cell carcinoma, all of which have diverse clinical behaviors. Until a few years ago, all histologic subtypes of NSCLC were treated with similar systemic therapy regimens. Though distinct differences in sites of metastasis, OS, and smoking behavior were recognized, there was no specific reason to use histology for selection of systemic therapy. The importance of distinguishing histology of NSCLC was realized with the development of new therapies that resulted in different toxicities and outcomes based on the histology. This effect was first realized in the phase II study of bevacizumab; the risk of pulmonary hemorrhage was predominantly seen in patients with squamous histology. Bevacizumab was subsequently restricted to patients with predominant nonsquamous histology. Several other antiangiogenic agents have also demonstrated a higher risk of bleeding with squamous histology, thus defining this as a class effect.
Pemetrexed was the first cytotoxic agent that has shown a clear correlation between histology and efficacy. Scagliotti et al. conducted a phase III study to compare the efficacy of cisplatin and pemetrexed with that of cisplatin and gemcitabine for patients with advanced NSCLC. The OS and PFS were similar for the study population, which included approximately 1700 patients. A preplanned subset analysis was conducted to study the outcomes for patients with nonsquamous histology. This revealed a significant improvement in survival for nonsquamous patients with the cisplatin-pemetrexed regimen (11.8 months vs. 10.4 months). Conversely, the cisplatin–gemcitabine regimen was superior in patients with squamous cell histology. Based on this study, and similar observations from other studies with pemetrexed, this agent is not considered appropriate for the treatment of squamous cell lung cancer.
In contrast, nab-paclitaxel, an albumin-bound formulation of paclitaxel, benefits patients with squamous cell lung cancer preferentially over nonsquamous tumors. In a phase III study of weekly nab-paclitaxel with carboplatin compared with standard paclitaxel and carboplatin given every 3 weeks, comparable OS was noted for the two regimens. The response rate was superior with nab-paclitaxel regimen for the overall patient population (32% vs. 25%). The response rate was higher with nab-paclitaxel in patients with squamous histology (response rate ratio 1.6890, 95% CI, 1.271–2.221, p < 0.001). There was no difference in overall response rate (ORR) in patients with nonsquamous histology between the two regimens (26% with nab-paclitaxel versus 25% with paclitaxel, p = 0.808). Nab-paclitaxel is a Food and Drug Administration approved therapy for advanced NSCLC in combination with carboplatin. It has the advantage of not requiring premedications needed with the standard formulation of paclitaxel. It is also associated with a lower incidence of grades 3–4 neuropathy compared with paclitaxel. The biologic reasons behind the correlation between histology and efficacy of pemetrexed and nab-paclitaxel are not known, and some exploratory hypotheses are described in a subsequent section of this chapter.
Management of Elderly Patients
In the United States, patients aged more than 65 years represent two-thirds of the lung cancer cases, and the median age at diagnosis is >70 years. Furthermore, nearly 15% of patients are over the age of 80 years at diagnosis. A number of physiologic functions, especially renal and hematopoietic functions, are altered with ageing that impact on chemotherapy tolerance and toxicity. Furthermore, elderly patients have more comorbid conditions compared with younger ones and are more likely to take prescription medications for other ailments, which may interfere with the pharmacokinetic disposition of anticancer agents. Comorbid conditions can be evaluated using the Charlson Comorbidity Index, or by the more detailed Cumulative Illness Rating Scale-Geriatric. In a Veterans Affairs Central Cancer Registry containing 20,511 NSCLC cancer patients from 2003 to 2008, the percentage of patients receiving guideline-recommended chemotherapy treatment decreased with increasing age. In an analysis of the SEER-Medicare database in the United States, only about 25% of the older patients received systemic chemotherapy for advanced stage disease. Furthermore, platinum-based regimens were given to less than 25% of the patients that received chemotherapy.
Until recently, the majority of the treatment recommendations for chemotherapy in elderly NSCLC patients were based on subset analyses of outcomes for elderly patients included in clinical trials for all patient age-groups. Elderly patients in these studies were likely to be highly selected based on functional status and might not be representative of the older patient population “at-large.” Moreover, there is a wide variation between studies regarding the definition of older patients across clinical trials. In earlier studies, age >65 years was often used to define older patients. In recent trials, >70 years has become the threshold for elderly patient-specific trials. Another noteworthy aspect is that many studies in the general lung cancer population limit entry to patients <75 years of age. For all these reasons, treatment decisions for older patients should be made based on available data, patient preferences, comorbid illness, and molecular status.
The role of chemotherapy exclusively in older patients was established in a phase III study that demonstrated superiority for vinorelbine over best supportive care (ELVIS study). The survival improvement was observed despite early closure of the study due to slow accrual and the fact that the necessary sample size had not been met. This was the first elderly patient–specific study in lung cancer to define the role for chemotherapy in advanced stage disease. Subsequently, a study that compared the combination of gemcitabine with vinorelbine versus both revealed no therapeutic advantage for the combination. These studies led to the adoption of single-agent chemotherapy as the standard approach for older lung cancer patients.
The role of combination regimens in older patients was not defined until recently. Subset analysis from a number of randomized trials demonstrated that outcomes for older patients enrolled in clinical trials were similar to that of younger individuals. In a study reported by Lilenbaum et al., treatment with carboplatin and paclitaxel was associated with an increase in response and survival compared with single-agent therapy with paclitaxel, but the differences did not reach statistical significance. A benefit of similar magnitude was observed for the combination compared with monotherapy in a subgroup analysis of patients >70 years, and there was no significant difference in survival between elderly patients and younger patients with carboplatin–paclitaxel. However, in a combined analysis of two SWOG trials, elderly patients (above 70 years) treated with platinum-based combinations had a shorter survival (7 months versus 9 months; ( p = 0.04) and experienced more frequent grade 3–5 neutropenias compared with younger patients.
Most studies addressing directly the benefit of chemotherapy to elderly patients demonstrated a survival benefit to some extent for patients treated with the more aggressive therapy arm ( Table 44.4 ). In a recent phase III study by Quoix et al., 451 patients aged 70–89 years with locally advanced or metastatic NSCLC and WHO performance status scores of 0–2 received either four cycles of carboplatin/paclitaxel or five cycles of vinorelbine or gemcitabine monotherapy. Second-line treatment was defined to be erlotinib for both arms. The study population had a median age of 77 years. The median OS was significantly superior for doublet chemotherapy (10.3 versus 6.2 months; HR 0.64, 95% CI, 0.52–0.78; p < 0.0001). The combination regimen was also associated with more hematologic and nonhematologic toxicity including neutropenia (48.4% vs. 12.4%) and asthenia (10.3% vs. 5.8%). This is the first prospective trial for elderly patients that demonstrated a survival benefit for combination chemotherapy. It is noteworthy that the study utilized a weekly schedule of paclitaxel with administration of carboplatin every 4 weeks, which appears to have a slightly favorable tolerability over the standard 3-weekly schedule. It is evident from this cumulative evidence that elderly patients with a good performance status are appropriate candidates for platinum-based chemotherapy. Also, consideration for primary prophylaxis with granulocyte colony stimulating factor might be appropriate for certain combination regimens in elderly patients. Given the potential detrimental effects of cisplatin on the kidneys and other end organs in older subjects, carboplatin-based regimens are preferred for the treatment of advanced NSCLC. For less fit patients, monotherapy might be suitable; however, one needs to balance possible benefits and risk followed by discussion with the patients about the role of chemotherapy.
| Author (year) | Number of Patients | Median Age (y) | Drugs | Response Rate (%) | Median Survival (Months) | 1-Year Survival Rate (%) | p |
|---|---|---|---|---|---|---|---|
| ELVIS (1999) | 76 85 | 74 | Vinorelbine BSC | 19.7 – | 6.5 4.9 | 32 14 | 0.03 |
| Frasci (2000) | 60 60 | 74 | Vinorelbine Gemcitabine + vinorelbine | 22 15 | 7 4.5 | 13 30 | <0.01 |
| Gridelli (2003) | 700 | 74 | Vinorelbine Gemcitabine Gemcitabine + vinorelbine | 21 16 18.1 | 8.5 6.5 7.4 | 42 28 34 | NS |
| Kudoh (2006) | 182 | 76 | Vinorelbine Docetaxel | 9.9 22.7 | 9.9 14 | NR NR | NS |
| Quoix (2011) | 226 225 | 77 | Vinorelbine or gemcitabine Carboplatin + weekly paclitaxel | 10 27 | 6.2 10.3 | 25.4 44.5 | 0.0004 |
Management of Patients with a Poor Performance Status
The performance status (PS) of the patient is an important prognostic factor in lung cancer. An ECOG PS of 2, alternatively termed “marginal PS” or “poor risk,” is defined as being “ambulatory and capable of all self-care but unable to carry out any work activities; up and about more than 50% of waking hours.” The designation of PS remains subjective, as evidenced by the discordance between physician assessments of performance status versus self-assessment by patients. Physicians tend to overestimate the performance status of patients in general.
Subset analyses of trials for patients with advanced NSCLC with eligibility ranging from PS 0 to 2 have historically shown that PS 2 patients experience a much shorter survival. Some studies have pooled PS 2 patients with the elderly, most commonly defined as being older than 70 years of age. Though decline in PS is often related to high disease burden related to lung cancer, it should be distinguished from poor PS related to comorbid illness. Until now, studies conducted in older patients have not adequately made this distinction, which makes it difficult to recommend aggressive therapies for those with poor PS. In recent years, several studies have been conducted exclusively for patients with a poor PS. The combination of carboplatin and paclitaxel was superior to single-agent paclitaxel (median survival of 4.7 vs. 2.4 months) in PS 2 patients in a prospectively specified subgroup analysis for patients with poor PS that were enrolled to the Cancer and Leukemia Group B (CALGB) trial. In another study for patients with a poor PS, the combination of carboplatin and paclitaxel was superior to single-agent therapy with erlotinib (9.7 months compared with 6.5 months with erlotinib). In a recent phase III trial for advanced NSCLC patients, patients with PS 2 were randomly assigned to a combination therapy of carboplatin–pemetrexed or pemetrexed alone. PS was determined by the treating physician and verified by another physician at each participating site. There was a statistically and clinically significant improvement with the doublet in response rate (23.8% vs. 10.3%; p = 0.032), progression-free survival (median, 5.8 vs. 2.8 months; HR, 0.46; 95% CI, 0.35–0.63; p < 0.001), and OS (median, 9.3 vs . 5.3 months; HR, 0.62; 95% CI, 0.46–0.83; p = 0.001). However, toxicity was also higher in the combination arm, with more treatment-related deaths (3.9% vs. 0%). This was the first prospective study to demonstrate benefit from platinum-based therapy in patients with poor PS. Future trials should address the impact of comorbid conditions versus cancer burden as the reason behind decline in PS for patients and its correlation to outcome with combination therapies.
Taking the recent data into account, patients with heavy disease burden and poor PS may also be offered combination therapy. As with any clinical decision, therapy selection is utterly dependent on the patient preference and physician judgment throughout the process of selecting palliative chemotherapy in the setting of advanced NSCLC. Molecular testing is strongly recommended even in patients with a poor PS, since the use of appropriate targeted therapy in selected patients has been reported to result in robust responses and improved physical function.
Biomarkers for Selection of Chemotherapy
The use of biomarkers to select appropriate chemotherapy regimens has long been a focus of research. Lung cancer cells have a relative deficiency of DNA repair machinery compared with normal cells; this makes them more sensitive to the DNA damaging effects of cytotoxic chemotherapy. Somatic excision repair cross-complementing gene 1 ( ERCC1 ) has been evaluated as a prognostic and predictive biomarker of response to treatment with platinum agents. Ribonucleotide reductase M1 ( RRM1 ) is the catalytic subunit of ribonucleotide reductase, which converts ribonucleoside diphosphates into deoxyribonucleosides in DNA synthesis. Since RRM1 is the primary target of gemcitabine, it has been studied as a predictive biomarker for efficacy of gemcitabine.
A randomized phase III study was conducted to utilize ERCC1 expression to personalize therapy in NSCLC. The hypothesis was to treat patients with ERCC1 -overexpressing tumor with nonplatinum regimens and those with low ERCC1 expression with platinum-containing regimens; 82.4% of patients randomized had adequate tissue for ERCC1 mRNA expression analysis; 444 patients with stage IIIB or IV NSCLC were then randomized in a 2:1 ratio to the genotypic arm, where patients were treated according to their ERCC1 status ( ERCC1 low patients with cisplatin and docetaxel and ERCC1 high patients with gemcitabine and docetaxel) or to the control arm, where all patients received cisplatin and docetaxel irrespective of biomarker status. The study achieved its primary end point of improvement in response rate with 50.7% in the genotypic arm compared with 39.3% in the control arm ( p = 0.02). However, since there was no significant improvement in survival in the genotype arm, this limited the utility of ERCC1 as a predictive biomarker. In another recently published phase III study, treatment was assigned based on tumoral expression of ERCC1 and RRM1 for patients with chemotherapy-naive advanced NSCLC. ERCC1 and RRM1 expression were determined by an automated quantitative analysis (AQUA) based on immunohistochemistry. Out of 275 eligible patients, 183 were randomized to the customized approach and 92 patients to control. There was no difference between the two groups in the primary end point of PFS, or the secondary outcomes of OS or response rate (11 months and 36.5% with customized therapy vs. 11.3 months and 38.8% in control arm, p = 0.66 for survival). In addition to these disappointing results, problems regarding the sensitivity of the antibodies used for detection of ERCC1 expression have recently been observed.
Pemetrexed exerts anticancer effects by inhibiting thymidylate synthase (TS), dihydrofolate reductase, and glycinamide ribonucleotide formyl transferase. TS is an enzyme that converts deoxyuridylate to deoxythymidylate that is necessary for DNA synthesis. Since TS is the main target of pemetrexed, low levels of TS have been hypothesized to predict increased response rate to pemetrexed therapy. In a study of 56 patients with NSCLC, TS mRNA and protein levels were higher in patients with squamous cell carcinoma. TS levels may explain why patients with squamous cell histology are resistant to pemetrexed compared with adenocarcinoma histology, though this remains to be confirmed.
Taxanes bind to β-tubulin and lead to stabilization of microtubules, resulting in apoptosis. High levels of β-tubulin have been associated with resistance to treatment with docetaxel and paclitaxel in cell lines. In 91 patients with advanced NSCLC who were treated with paclitaxel (47) or nontaxane regimens (44), those with low expression of class III β-tubulin by immunohistochemistry (IHC) had improved response rates, PFS, and OS when treated with paclitaxel. In a meta-analysis of 552 patients in 10 studies examining either paclitaxel or vinorelbine containing regimens, decreased expression of class III β-tubulin was associated with improved OS (HR, 1.40; p < 0.00001). These observations have not been confirmed in prospective studies and the role of β-tubulin as a predictive marker for taxanes still remains unproven.
Combination of Targeted Agents with Platinum-Based Chemotherapy
The development of molecularly targeted agents has led to the evaluation of several novel compounds in combination with platinum-based chemotherapy for first-line treatment of advanced NSCLC. These studies were usually supported by supra-additive or synergistic preclinical interactions between these agents. However, the vast majority of these studies failed to demonstrate an improvement in survival with the addition of a targeted agent to standard chemotherapy. These studies were typically conducted in unselected patient populations and did not include efforts to identify predictive biomarkers. More recently, studies with novel combinations are focused on identifying a subset of patients that might derive robust benefits. The first combination strategy to demonstrate survival benefit was the addition of bevacizumab, a monoclonal antibody against the VEGF, to standard chemotherapy.
Antiangiogenic Therapy
It has been widely observed that for tumor development and growth to proceed beyond a defined volume, the development of a new blood supply is necessary. Therefore, most solid tumors need the formation of new blood vessels for continued growth and metastasis, which may be achieved by the induction of endothelial cell sprouting from the preexisting vasculature (so-called angiogenesis). The monoclonal anti-VEGF antibody bevacizumab, which blocks the binding of VEGF to its high-affinity receptors, was the first angiogenesis inhibitor to complete clinical development and is currently the only antiangiogenesis agent approved for the treatment of lung cancer.
In a phase II trial in 99 unselected NSCLC patients, a higher response rate (31.5% vs. 18.8%), longer median time to progression (7.4 vs. 4.2 months), and a modest increase in survival (17.7 vs. 14.9 months) was observed for treatment with carboplatin and paclitaxel plus bevacizumab (15 mg/kg) compared with the chemotherapy control arm. However, 9% of the patients treated with bevacizumab experienced life-threatening pulmonary hemorrhage (PH), which was fatal in four patients. Since the majority of the patients with hemoptysis had squamous cell histology, tumor cavitation, and disease location close to major blood vessels, these clinical situations were excluded in subsequent studies.
Two large clinical trials demonstrated efficacy of bevacizumab in combination with a platinum containing chemotherapy in patients with advanced NSCLC of nonsquamous histology, resulting in the FDA approval for this setting ( Table 44.5 ). In the ECOG 4599 study, a substantial clinical benefit for NSCLC patients treated with 15 mg/kg bevacizumab plus carboplatin and paclitaxel versus chemotherapy alone was seen (HR, 0.66 for PFS with a median of 6.2 vs. 4.5 months; HR, 0.79 for OS with a median of 12.3 vs. 10.3 months). These results were partly confirmed by another large phase III trial where NSCLC patients had an improved PFS with the addition of low-dose bevacizumab (7.5 mg/kg; HR, 0.75 (0.64–0.87); p = 0.0003) or high-dose bevacizumab (15 mg/kg; HR, 0.85 (0.73–1.00); p = 0.0456) to a standard chemotherapy of cisplatin and gemcitabine. In the later study, however, the median net gain of PFS was relatively modest with bevacizumab, and, more importantly, there was no improvement in OS (HR, 0.93; 95% CI, 0.78–1.11; p = 0.42 and HR, 1.03; 95% CI, 0.86–1.23; p = 0.761 for the 7.5 and 15 mg/kg groups, respectively). As a result of these phase III trials in chemotherapy-naive NSCLC patients, bevacizumab has been approved for the treatment of advanced NSCLC, excluding predominantly squamous cell histology, in combination with platinum containing chemotherapy.
| ECOG 4599 | AVAiL | |
|---|---|---|
| Regimen | Carboplatin/paclitaxel/± bevacizumab | Carboplatin/gemcitabine/± bevacizumab |
| Bevacizumab Dose | 15 mg/kg | Low dose: 7.5 mg/kg High dose: 15 mg/kg |
| Response Rate | 35% vs. 15% | 34% (low) vs. 30% (high) vs. 20% |
| Median Progression-Free Survival | 6.2 months vs. 4.5 months | 6.7 months (low) vs. 6.5 months (high) vs. 6.1 months |
| Median Overall Survival | 12.3 months vs. 10.3 months | 13.6 months (low) vs. 13.4 months (high) vs. 13.1 months |
| Hazard Ratio Death | 0.79 (95% CI, 0.67–0.92), p = 0.003 | 0.93 (95% CI, 0.78–1.11), p = 0.42 (low) 1.03 (95% CI, 0.86–1.23), p = 0.76 (high) |
Bevacizumab and other antiangiogenic agents are associated with a low, but significant risk of grade ≥3 or fatal (grade 5) pulmonary hemorrhage. Two meta-analyses have found that the use of bevacizumab in combination with chemotherapy for the treatment of various tumor types conferred a significantly increased risk of severe and fatal bleeding events and treatment-related mortality versus chemotherapy alone. However, patients with NSCLC are at an increased risk of pulmonary hemorrhage owing to the underlying disease process, with non–life-threatening bleeds occurring in 16% of 877 patients with lung cancer. Nearly 3% of these were fatal. Massive pulmonary hemorrhage was significantly associated with squamous cell tumors, cavitation, and with bronchial (vs. peripheral) tumors. Recently, an expert panel recommended that patients with squamous histology and/or a history of grade ≥2 hemoptysis (≥2.5 mL per event) should not receive bevacizumab. However, no clinical or radiologic features (including cavitation and central tumor location) reliably predict severe pulmonary hemorrhage in bevacizumab-treated patients. Major blood vessel infiltration and bronchial vessel infiltration, encasement, and abutting may increase the risk of pulmonary hemorrhage; however, standardized radiologic criteria for defining infiltration have not been established. In all these studies, patients received maintenance therapy with bevacizumab after completion of chemotherapy; still, the benefit of this maintenance has not been prospectively addressed in large clinical trials for NSCLC, so far.
Other Antiangiogenic Agents
The therapeutic success achieved with bevacizumab in NSCLC has prompted the evaluation of several other antiangiogenic agents. Various small-molecule tyrosine kinase inhibitors (TKIs) have been tested in clinical studies in advanced NSCLC. As a common feature of these agents, the spectrum of inhibited receptors is not limited to VEGF receptor (VEGFR) but comprises various further growth factors and signaling pathways. While the broader activity was considered likely to improve the therapeutic benefit, the spectrum of side effects is also expanded, and it renders difficult conclusions regarding the relevance of targeting VEGFR. To date, no drug in this class has demonstrated survival improvement in randomized studies. Phase II studies addressing monotherapy with multi-TKIs have demonstrated modest activity with response rates ranging from 7% to 10% and median time to progression from 2.4 months to 5.8 months in pretreated NSCLC patients. Moreover, several phase III trials have recently been completed that have assessed multikinase antiangiogenic TKIs in a second-, third-, and/or fourth-line setting such as sunitinib (in combination with erlotinib), vandetanib (in combination with docetaxel or pemetrexed), and sorafenib monotherapy. Unfortunately, the outcome of these trials was disappointing; despite an improvement in response rates and PFS in most trials, these antiangiogenic TKIs had no impact on OS. Some additional phase III trials are ongoing to assess novel newer agents that have shown promising activity in phase II trials such as pazopanib and apatinib.
In a recent meta-analysis of 15 randomized controlled trials investigating multitargeted antiangiogenic TKIs (vandetanib, sunitinib, cediranib, sorafenib, motesanib) in combination with chemotherapy or as monotherapy in advanced NSCLC, treatment with multi-TKIs was associated with a significantly longer PFS (HR, 0.824; 95% CI, 0.759–0.895; p < 0.001) and superior response rate (OR, 1.27; 95% CI, 1.13–1.42; p < 0.0001) compared with the control arm. However, OS was not significantly different (HR, 0.962; 95% CI, 0.912–1.015; p = 0.157). Other VEGFR inhibitors currently under development include pazopanib, apatinib, and nintedanib.
Two phase III trials (LUME-Lung 1 and 2) were presented investigating nintedanib, a TKI targeting VEGF-, PDGF-, and FGF-receptors. After failure of first-line chemotherapy, 1314 stage IIIB–IV NSCLC patients were randomized to treatment with docetaxel with or without nintedanib (LUME-1). The primary end point, PFS, was significantly improved by the addition of nintedanib (3.4 vs. 2.7 months; HR, 0.79; 95% CI, 0.68–0.92; p = 0.0019) while OS was not significantly different (10.1 vs. 9.1 months; HR, 0.94; 95% CI, 0.83–1.05; p = 0.2720). However, in a prespecified subgroup analysis, patients with adenocarcinoma had both a significant and clinically meaningful improved PFS (4.0 vs. 2.8 months; HR, 0.77; 95% CI, 0.62–0.96; p = 0.0193) and OS (12.6 vs. 10.3 months; HR, 0.83; 95% CI, 0.70–0.99; p = 0.0359). Also, there was a significant improvement in disease control rate with the nintedanib plus docetaxel combination (adenocarcinoma 60.2% vs. 44%; OR, 1.93; p < 0.0001; squamous cell carcinoma 49.3% vs. 35.5%; OR, 1.78; p < 0.0009). These data were somewhat confirmed by another phase III trial investigating the second-line combination of pemetrexed with or without nintedanib in patients with advanced or recurrent, nonsquamous NSCLC after treatment with first-line chemotherapy (LUME-2). Despite stopping the trial after recruitment of 713 of the 1300 intended patients, the primary end point was still met as PFS was significantly superior after treatment with nintedanib plus pemetrexed (4.4. vs. 3.6 months; HR, 0.83; 95% CI, 0.70–0.99; p = 0.0435). Collectively, the LUME-1 study is the first phase III study demonstrating a survival benefit of adding a multi-TKI to chemotherapy in a prespecified subgroup.
Ramucirumab is a monoclonal antibody that binds to VEGFR-2 and blocks ligand binding and activation. A phase III study (REVEL) investigated ramucirumab in combination with docetaxel as second-line therapy after failure of platinum-based therapy ( n = 1253 patients including those with squamous histology). The addition of ramucirumab to docetaxel resulted in a significant improvement in median OS (10.5 months vs. 9.1 months; HR, 0.86; 95% CI, 0.75–0.98; p = 0.023) and PFS (4.5 months vs. 3.0 months; HR, 0.76; 95% CI, 0.68–0.86; p < 0.0001). Notable toxicities with ramucirumab were neutropenia, febrile neutropenia, fatigue, leukopenia, and hypertension. The combination of docetaxel with ramucirumab has gained approval by the United States FDA for salvage therapy of advanced NSCLC. Aflibercept is an investigational recombinant protein composed of epitopes of the extracellular domains of human VEGFR fused to the constant region (Fc) of human immunoglobulin G1 (IgG1) functioning as a soluble decoy receptor. The addition of aflibercept to second-line docetaxel in NSCLC patients was associated with improved ORR, but there was no improvement in OS. Vascular disrupting agents that cause destruction of existing tumor vasculature have also been studied in lung cancer without much success. There are no proven biomarkers to select patients for antiangiogenic agents in NSCLC. This has undoubtedly limited the utilization of these agents in the clinic.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree