Fig. 1.1
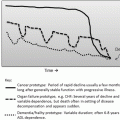
Two conceptualizations of frailty. (a) Phenotypic frailty. Phenotypic frailty is conceptualized as a clinical syndrome driven by age-related biologic changes that drive physical characteristics of frailty and eventually, adverse outcomes. (b) Deficit accumulation frailty. The deficit model of frailty proposes that frailty is driven by the accumulation of medical, functional, and social deficits, and that a high accumulation of deficits represents accelerated aging. An important distinction between these two conceptualizations of frailty is that biologic driven frailty causes the physical characteristics of frailty (arrows pointed outward). In contrast, deficit accumulation frailty is caused by accumulated abnormal clinical characteristics (arrows pointed inward) (Adapted from Journal of the American College of Surgeons, Volume 221, Issue 6, Robinson TN, Walston JD, Brummel NE et al., Frailty for Surgeons: Review of a National Institute on Aging Conference on Frailty for Specialists, 1083–1092, Copyright 2015, with permission from Elsevier.)
Another major theoretical construct for frailty comes from Rockwood et al., who conceptualized frailty as an aggregate of illnesses, disability measures, cognitive and functional declines that has been termed deficit-driven frailty [9]. According to this model, the more deficits or conditions that an individual has, the more frail the individual is. In this agnostic approach , almost any conditions or deficits are interchangeable in index tools. This conceptual basis has also been widely utilized to develop risk assessment tools that tally a broad range of comorbid illnesses , mobility and cognitive measures, and environmental factors to capture frailty. Although this concept of deficit-driven frailty has been utilized in many population studies to assess risk for mortality and other adverse health outcomes, biological and intervention studies have been more difficult because of non-specificity in the hypothetical origin in this measure of frailty [10].
Beyond these two approaches, over 70 frailty measurement tools have been cited in the literature [11]. Most have been developed and validated in research population databases. Many have been developed through adaptations to either the phenotypic/physical frailty approach or the index/deficit approach or combinations of the two. Others have been developed to have a cognitive focus. Despite the proliferation of assessment tools in the literature, acceptance of a standardized definition for frailty in clinical practice has been slowed by the broad heterogeneity in measures that include medical, social, cognitive, psychological, and educational factors [12, 13]. Considerations related to chronological age, comorbidities, and disability, while associated with frailty, have also led to lack of consensus of frailty measurement [1, 13–15]. Despite this, many tools are usable for risk assessment and many are being developed for use in disease specific populations such as chronic kidney disease, transplantation candidates, or vascular surgery.
Finally, given the high prevalence of cognitive decline later in life, it is important to consider its potential role in frailty. Frailty is highly associated with an increased risk of mild cognitive impairment and an increased rate of cognitive decline with aging [16, 17]. Conversely, the presence of cognitive impairment increases the likelihood of adverse health outcomes in older adults who meet criteria for physical frailty. Hence, it may be considered an additive risk factor to frailty in those older adults with both conditions.
1.3 Frailty Prevalence, Epidemiology, and Risk
Dozens of population studies of frailty have been developed in the past 15 years [11]. Many have used physical/syndromic frailty or index/deficit type of frailty measures or derivatives to determine the demographics and epidemiology of frailty. Although the prevalence of frailty varies with the tool used to define frailty and with the population studied, most population studies performed in the USA and Canada have estimated that the prevalence of frailty lies between 4 and 16 % in men and women aged 65 and older [1, 18–21]. A large review study using physical frailty measured in 15 studies that included 44,894 participants identified a prevalence of frailty of 9.9 %; when psychosocial aspects were included in the definition, prevalence was 13.6 % among eight studies that included 24,072 participants [22]. Prefrail individuals, generally identified with a physical frailty type tool , are more common in these population studies, with prevalence ranging from 28 to 44 % [1, 20, 21].
As to clinical transition towards frailty, most of the studies have been performed using the physical frailty phenotype. For example, in a study in the USA of nearly 6000 community-dwelling men aged 65 and older, at an average follow-up of 4.6 years, 54.4 % of men who were robust at baseline remained robust, 25.3 % became prefrail, and 1.6 % became frail. The remaining subjects were accounted for by 5.7 % mortality and the remaining 13 % were lost to follow-up [21]. Of those individuals who were prefrail, over 10 % went on to become frail over the next 3 years.
Demographic associations with frailty include older age [20], lower educational level [20], smoking, unmarried status, depression, and African American or Hispanic ethnicity [10, 21, 23]. A number of chronic disease states, including most especially congestive heart failure, diabetes mellitus, hypertension, and peripheral artery disease [14, 24, 25] are also significantly associated with physical frailty.
Frailty has been widely utilized as a mortality risk assessment tool . Several studies have compared the most commonly utilized screening tools and found that these indices were comparable in predicting risk of adverse health outcomes and mortality [18, 26, 27]. A 2013 consensus conference also referenced tools that can be easily utilized to diagnose frailty [28]. In most studies of physical frailty, the increasing mortality in models adjusted for disease, age, and socioeconomic factors ranges from 2.24 at 3 years in the Cardiovascular Health Study to 6.03 in the Women’s Health and Aging Studies 1 and II [1, 19]. In the longitudinal Women’s Health Initiative Observational Study, mortality risk was increased over 3 years in those with baseline frailty (HR 1.71; 95 % CI 1.48–1.97) [20]. In a study in men, mortality was twice as high for frail, compared with robust, men (HR 2.05; 95 % CI 1.55–2.72) [21]. Mortality prediction was demonstrated to be similar across 8 scales of frailty developed within previously collected data in the Survey of Healthy, Aging and Retirement in Europe (SHARE) , with death rates three to five times higher in cases classified as frail compared with those not classified as frail in all tools studied [29]. This collective evidence suggests that those who are frail have a 2–6 fold risk of mortality in the subsequent 3 years compared to their robust counterparts.
In addition to mortality, frailty status is predictive of a host of adverse health outcomes. After adjustment for comorbidities, frailty predicted hip fractures (HR 1.74 (1.37–2.22) and disability (HR 5.44 (4.54–6.52) over 3 years in the participants of the Women’s Health Initiative [20]. Frailty also predicted adverse outcomes related to renal transplantation, general surgery interventions, and trauma [30, 31].
In surgical populations, frailty predicts adverse outcomes as well. Using a frailty phenotype tool to ascertain frailty, this group measured frailty in a preoperative assessment and found that the frail individuals were at increased risk of postoperative complications (OR 2.54; 95 % (I 1.12–5.77), increased length of stay (incidence ratio 1.69; 95 % (I 1.28–2.23), and a markedly increased risk of discharge to an institutional care setting such as rehabilitation or nursing home (OR 20.48; 95 % (I 5.54–75.68).
1.4 Pathophysiology
There is increasing evidence that dysregulated immune, endocrine, stress, and energy response systems are important to the development of physical frailty. The basis of this dysregulation likely relates to molecular changes associated with aging, genetics, and specific disease states, leading to physiologic impairments and clinical frailty (Fig. 1.2) [7]. Sarcopenia , or age-related loss of skeletal muscle and muscle strength , is a key component of physical frailty. Decline in skeletal muscle function and mass is driven in part by age-related hormonal changes [32–35] and increases in inflammatory pathway activation [36].
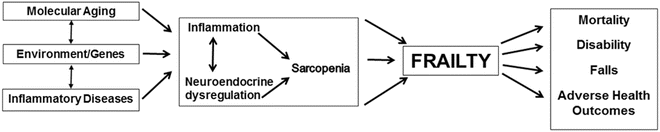

Fig. 1.2
Potential biological etiologies that drive physical frailty and the vulnerability to adverse health outcomes
Multiple age-related hormonal changes have been associated with frailty. Decreased growth hormone and insulin-like growth factor-1 levels in later life (IGF-1) [32, 37, 38] are associated with lower strength and decreased mobility in a cohort of community-dwelling older women [39]. Decreased levels of the adrenal androgen dehydroepiandrosterone sulfate (DHEA-S) [32] are also lower in frail older adults. DHEA-S plays an important role in maintaining muscle mass and indirectly prevents the activation of inflammatory pathways that also are a component of frailty [40]. Chronically increased cortisol levels [41], especially in the afternoon, are common in frailty and likely impact skeletal muscle and immune system function. Evidence is mixed that lower levels of the reproductive hormones estrogen and testosterone contribute to frailty [42–45]. However, there is stronger evidence that links decreased 25(OH) vitamin D [46] levels to frailty [47, 48].
There is strong evidence linking chronic inflammatory pathway activation to frailty. Serum levels of the proinflammatory cytokine IL-6 and C-reactive protein (CRP), as well as white blood cell and monocyte counts, are elevated in community-dwelling frail older adults [32, 46, 49, 50]. IL-6 acts as a transcription factor and signal transducer that adversely impacts skeletal muscle, appetite, adaptive immune system function, and cognition [51] and contributes to anemia [52, 53]. Immune system activation may trigger the clotting cascade, with a demonstrated association between frailty and clotting markers (factor VIII, fibrinogen, and D-dimer) [49]. Further, there is evidence linking a senescent immune system to chronic CMV infection and frailty [54]. Frail older adults are also less likely to mount an adequate immune response to influenza vaccination, suggesting a biological driver of frailty [55].
Vaccine failure may contribute to the increased vulnerability to influenza and higher levels of influenza infection observed in frail older adults. Finally, there is increasing evidence linking dysregulation in stress response systems to frailty beyond the inflammatory and cortisol component detailed above. For example, dysregulation of the autonomic nervous system [56] and age-related changes in the renin-angiotensin system and in mitochondria likely impact sarcopenia and inflammation, important components of frailty [57]. This dysregulation in stress response systems may be especially relevant to patients undergoing stress surgical procedures, and likely contributes to markedly increased risk of adverse outcomes in frail patients.
1.5 Clinical Assessment of Frailty
Clinical practitioners are increasingly interested in frailty, its definitions, and most importantly how it can be utilized to reduce risk of adverse outcomes and to improve the healthcare of older adults. Although no gold standard has emerged to measure frailty or on how best to use information on frailty once it is obtained, many research and clinical practice groups are moving toward incorporation of frailty measurements into clinical practice. Indeed, the identification of frailty in any clinical practice settings may be helpful in highlighting the need for additional assessment and the need for individualized treatment plans that reduce risk. As part of a movement to incorporate frailty measures into clinical practice, a consensus group of delegates from international and United States societies related to Geriatrics and Gerontology recently recommended that all persons over age 70, those adults with multiple chronic disease states or weight loss exceeding 5 % over a year should be screened for frailty. No one tool was recommended for frailty screen, although several currently available tools described below were highlighted for potential use [58].
1.6 Choosing a Specific Frailty Tool
Few guidelines exist on how to best choose a frailty assessment tool, although a recent publication outlines how most tools have been utilized to date [11]. This is in part because most frailty assessment tools have not been extensively validated or utilized across populations, and few comparison studies have been done that show clear benefit of using one tool over the other. In addition, different tools may or may not be good matches to the intended use. For example, a brief screening tool may be appropriate for risk stratification and decision making related to whether or not to pursue a treatment option. However, a more formal frailty assessment tool that includes physical measurements such as walking speed or grip strength might be required to better define potentially helpful preoperative interventions.
Given the wide array of tools and the wide variety of populations in which the tools may need to be implemented, the choice of which assessment tool to use should be tailored to a clinical situation and clinical need. Choosing a tool that has been previously used in a variety of populations and that has demonstrated predictive validity in several settings should also influence the choice of tools. Considerations of available time in a busy clinical practice may also drive the decision process.
Although not yet available, the development of discipline-specific frailty assessment tools, along with specific clinical guidelines of how best to manage frail older adults after they are identified is of crucial importance as older and more frail individuals are considered for medical and surgical interventions . A recent NIA conference on frailty in clinical practice has helped to formalize recommendations in a variety of clinical settings. The following list of frailty measurement tools , used mostly in the past for risk assessment in population studies, and rationale for their use was recently reviewed by Robinson et al. [59].
1.6.1 Single Item Surrogate Frailty Assessments (2–3 min)
Because of the need for quick and efficient frailty ascertainment in a busy clinical setting, single item measurement tools have been proposed to stand in for a more formal frailty measurement. For example, gait speed measured over a 4 m distance, one of the five measured factors in the physical frailty phenotype assessment discussed below, is recognized as a highly reliable single measurement tool that predicts adverse outcomes [60, 61]. The inability to rise from a chair, walk 10 feet, turn around, and return to sitting in the chair in ≥15 s, often termed the timed up and go test, is closely related to both postoperative complications and 1-year mortality [59]. Some of these single measures are components of both the frailty index and frailty phenotype approaches, and although they can be easy to use and predictive of adverse outcomes, they lack sensitivity and specificity of the full frailty assessment tools .
1.6.2 Frail Scale and Study of Osteoporotic Fractures (SOF) Frailty Tool (<5 min)
The Frail Scale was developed as a quick screening tool for frailty and is loosely based on the physical frailty phenotype construct with an additional comorbidity question [62–64]. The Geriatric Advisory Panel of the International Academy of Nutrition and Aging advocates this approach for develop frailty as a case-finding tool [60]. It requires asking five questions and scoring a one for each yes (Table 1.1). Those who are frail score 3, 4, and 5; those who are robust score 0 [63]. The assessment is easy to perform and score, requires no extra measuring device, and has been found to identify those at most risk for adverse outcomes in populations.
Table 1.1
Frail scale questionsa
Fatigue | Are you fatigued? |
Resistance | Can you climb 1 flight of stairs? |
Ambulation | Can you walk 1 block? |
Illnesses | Greater than 5 |
Loss of weight | Greater than 5 % |
Another easy to use screening tool for quick risk assessment is the Study of Osteoporotic Fractures (SOF) frailty tool [26]. Frailty is determined when individuals have two of the following three components.
Weight loss of 5 % in the last year
Inability to rise from a chair five times without the use of arms, or
A “no” response to the question “Do you feel full of energy?”
Both of these tools can be readily deployed in a clinical setting as a way to find high risk patients who may need further assessment.
1.6.3 Physical or Phenotypic Frailty (10 min)
Phenotypic or physical frailty is widely used by frailty researchers and has been widely adapted to measure frailty in many clinical and research settings . As described above in the conceptual basis of frailty, it was designed around the concept of an aggregate loss of function across physiological systems, which is in turn manifested by specific signs and symptoms in frail older adults [1, 8]. This was then operationalized into a clinical exam described below. The tool has been widely validated to predict risk for adverse health outcomes as well as most frailty assessment tools in many different research and clinical settings. It has been especially prominent in the study of the biological basis of frailty, and in the development of interventions focused on the specific components of frailty [65, 66]. This frailty assessment tool was 1 of 2 strategies recognized by the American College of Surgeons/American Geriatric Society’s optimal preoperative assessment of the older adult [67]. Although the tool requires a questionnaire, a hand-held dynamometer, and a stopwatch in order to assess for frailty, it takes less than 10 min to perform by a trained clinician/technician. The recent development of comprehensive instructions and a web-based calculator for this tool has made it easier to use and has further reduced the time that it takes to get a frailty score. Access to needed measurement equipment, training guides, and the web-based calculator is available at http://hopkinsfrailtyassessment.org (December 23, 2015).
This clinical phenotype has five components that can be assessed using readily available measurement equipment and a web-based frailty calculator as described below. The score is determined on a 0–5 scale with 0 being not frail; 1–2 prefrail; and 3–5 frail. The severity of the risk is linear.
The major measurement domains include:
- 1.
Shrinking (greater than 5 % loss of body weight in the last year).
- 2.
Weakness (grip strength of the dominant hand in the lowest 20 % of the age and body mass index (BMI).
- 3.
Poor endurance (self-reported exhaustion).
- 4.
Slowness (lower 25 % of population average measures 4 m walking time).
- 5.
Low activity (assessed by activity questions that identify weekly energy expenditure of less than 383/270 Kcals for males and females, respectively).
1.6.4 Deficit Accumulation or Frailty Index
The most widely recognized deficit accumulation method to measure frailty was developed from the Canadian Health and Aging Study [68].
Between 21 and 70 deficits or comorbidities have been published and recommended for use in this assessment [68, 69]. Although considerable time may be needed to gather information on individual patients and set up an algorithm in a medical record, a frailty index score can be quickly and automatically generated once the electronic record is in place. The frailty index score is calculated as the number of characteristics that are abnormal (or “deficits”) divided by the total number of characteristics measured. Scoring has mostly been done by summing the total deficits and comparing to a published cut-off score , or by calculating a ratio between deficits and total number of characteristics. This tool can be accessed in a series of references [69–71] or through the link biomedgerontology.oxfordjournals.org/content/62/7/722.long (December 23, 2015).
1.6.5 Frailty Index Adaptations
Recent adaptations of index-type tools for risk assessment in a variety of clinical settings have been developed. These uses include risk assessment in older trauma patients and in HIV infected individuals [72, 73]. Given that no physical measurements are necessary to calculate an index score, hospitalized and non-ambulatory patients can be assessed using historical data gathered from medical records and perhaps family members. This makes these tools especially valuable for prognostication, and risk assessment for outcomes. Strength of these types of tools includes the fact that each is more specificity related to the condition than other more general tools, which in turn may allow for improved risk assessment and eventually guideline development. However, screening for frailty after acute illness or injury does not facilitate prehabilitation or other risk reduction techniques that may predate hospitalization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree