Plasminogen Activators
Six PAs have been approved by the US Food and Drug Administration (FDA) for use in major thrombotic diseases, namely, streptokinase (SK),
18 urokinase (UK),
19,
20 alteplase (t-PA),
21,
22 anistreplase (anisoylated plasminogen streptokinase [APSAC]),
23 reteplase,
24 and tenecteplase (TNK-t-PA),
25 although UK is no longer available in the United States, and anistreplase is rarely utilized
(Table 112.2). Certain PAs are considered relatively fibrin selective (e.g., rt-PA and derivatives, staphylokinase, UK) and digest clot with less systemic plasminogen activation, while nonfibrin selective agents (e.g., SK, APSAC) activate systemic and fibrin-bound plasminogen relatively indiscriminately.
26,
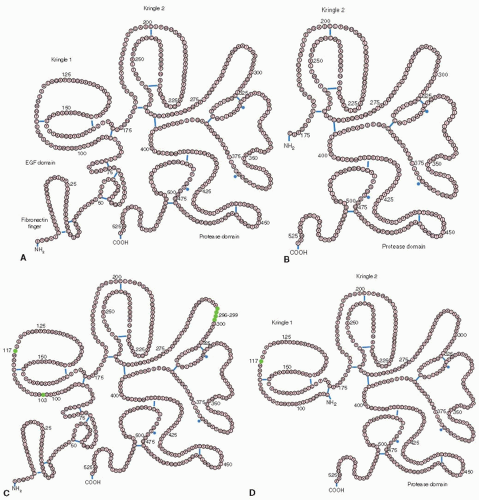
27The t-PA molecule contains four domains, an NH
2-terminal region which is homologous with the finger domains of fibronectin, an epidermal growth factor (EGF) domain, two kringle regions that are homologous with those of plasminogen, and a serine protease domain. Fibrin binding is mediated by the finger and kringle 2 domains,
in vivo clearance is regulated by the finger and growth factor domains and by the carbohydrate side chains, and plasminogen activator inhibitor-1(PAI-1) inhibition occurs via interaction with the positively charged amino acid sequence at position 296 to 304.
21,
22,
27Tissue-type PA variants have been synthesized by recombinant DNA technology in attempts to attain improved properties such as greater activity and fibrin specificity, prolonged half-life, or an attenuated lytic state
(FIGURE 112.2). Reteplase is a single-chain nonglycosylated deletion variant consisting only of the kringle 2 and the proteinase domain of human rt-PA and has a prolonged half-life (15 minutes) that allows for administration in a double bolus regimen.
28 Tenecteplase
25 is altered at three t-PA amino acid sites, conferring resistance to inhibition by PAI-1
29 and a prolonged half-life, allowing administration as a single bolus injection.
30 Lanoteplase (nPA) has a deletion of the finger and EGF domains and the carbohydrate at amino acid 117, resulting in a very prolonged half-life.
31 Vampire bat salivary PA is 70% homologous to t-PA, differing in that it has one kringle domain rather than two,
32,
33 conferring greater fibrin specificity. A recombinant version of the protein, desmoteplase, has been tested in a phase III trial of acute ischemic stroke.
34UK is secreted as a 54-kDa single-chain molecule (scuPA; pro-UK)
35,
36 and converted to a two-chain form (tcuPA) by proteolytic cleavage.
37 It contains an NH
2-terminal growth factor domain, a protease domain, and one kringle structure homologous to those of plasminogen and t-PA. In the presence of a fibrin clot, scuPA, but not tcuPA, induces fibrin-specific clot lysis.
38,
39,
40,
41Staphylokinase (Sak) is a PA produced by
Staphylococcus aureus strains that forms a 1:1 stoichiometric complex with plasmin that activates thrombus-bound plasminogen.
42,
43 As with SK, Sak is immunogenic, and patients may develop neutralizing antibodies.
44The major distinctions between PAs relate to their origin (antigenicity), half-life, potential for inducing a lytic state, and hemorrhagic potential
(Table 112.3). PAs that are derived from a human protein (UK, alteplase, reteplase, saruplase, tenecteplase, lanoteplase) are minimally or nonantigenic, whereas those from a bacterial species, whether purified from the
Streptococcus as SK, complexed with human plasminogen as anistreplase, or prepared
by recombinant technology from
Staphylococci as Sak,
45 can induce antibody formation and an allergic response. Modified recombinant forms of Sak and SK with lessened antigenicity and retained thrombolytic potency have been developed.
46,
47The half-life of each PA determines whether it can be administered as a bolus injection, short infusion, or continuous infusion. The most suitable agents for bolus injections are anistreplase (half-life, ˜40 minutes),
48 reteplase,
49 tenecteplase,
50 and lanoteplase,
51 and the least suitable is alteplase (half-life, 5 minutes),
52 which best utilizes a continuous infusion for therapy. Clinical results suggest that some of the newer agents will have less of a plasma lytic state, for example, tenecteplase,
53 but to date, all PAs are associated with a significant bleeding risk. The rate of bleeding is roughly equivalent for all agents, except for the higher rate of intracranial hemorrhage (ICH) with t-PA
54 or t-PA mutant derivatives,
49,
55 relative to results using SK. The anticipation of greater safety with a new PA, based on biochemical studies, must be considered cautiously, because no PA has yet been shown to be free of hemorrhagic risk.
Recombinant forms of UK,
35 saruplase (pro-UK, scu-PA),
36 Sak,
37 and BAT-PA
33 (from the salivary gland of
Desmodus rotundus), chimerics of t-PA and pro-UK,
56 and bifunctional agents composed of antifibrin or antiplatelet antibodies complexed to PA
57 are at various stages of clinical testing. Desmoteplase has marked homology with rt-PA and a much longer half-life of 21 minutes,
58 but it showed no clinical benefit and increased ICH (3.5% to 4.5%) in acute stroke patients at 3 to 9 hours after symptom onset.
34 A recombinant plasminogen that is activated by thrombin, rather than by a PA, has been developed as an agent that would work only at sites of fresh thrombus formation.
59 It has an injection half-life of >4 hours, and early studies have not shown increased ICH.
60,
61
Direct-Acting Thrombolytics
An old agent has recently been revisited as a therapeutic thrombolytic: the direct-acting thrombolytic enzyme plasmin.
62,
63,
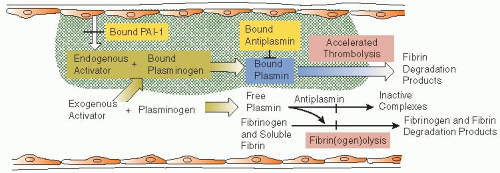
64 The potential for safety is based upon regional administration of the agent by catheter. PAs are administered in sufficient concentration that the inhibitory capacity of PAI-1 is overwhelmed, so they lyse hemostatic plugs and cause hemorrhagic complications, even after local (catheter) delivery
(FIGURE 112.3, middle-left and bottom-left panels). In contradistinction, plasmin is rapidly and irreversibly neutralized by α
2-antiplasmin,
65 so it lacks thrombolytic activity upon systemic infusion
(FIGURE 112.3, middle right). However, plasmin binds to and dissolves thrombus when administered by catheter, and, after thrombolysis, plasmin would be quickly neutralized by α
2-antiplasmin, thereby preventing its circulation to sites of vascular injury, and avoiding bleeding
that could result from hemostatic plug degradation.
66,
67 Animal studies support this hypothesis, showing that more than sixfold the therapeutic dose of plasmin does not induce bleeding, whereas t-PA causes bleeding at the therapeutic dose, and even at dosages as low as 25% of the optimal therapeutic amount.
68 Thrombolysis under conditions of limited plasminogen content (restricted blood flow) is superior following plasmin compared with t-PA.
66 Plasmin is safe and efficacious in preclinical models of cerebral artery occlusion,
69 has been assessed in patients with thrombosed hemodialysis shunts,
70 and is currently in clinical trial in patients with peripheral arterial or graft occlusion (NCT 00418483)
71 and acute ischemic stroke (NCT 01014975).
72Other direct-acting thrombolytic agents are shown schematically in
FIGURE 112.4.
63 A truncated plasmin, “miniplasmin,” consists of the plasmin serine protease domain and kringle 5
73,
74 and is inhibited by α
2-antiplasmin 100 times slower than is plasmin; thus its principal inhibitor is α
2-macroglobulin.
75,
76 Miniplasmin has shown efficacy in a canine femoral artery thrombolysis model
77 and in the middle cerebral artery ligation stroke model in mouse and hamster,
78 but there are no reports of this agent in human clinical trial.
63 Microplasmin is produced chemically by cleavage of the kringle domains from the serine protease domain
79 or by recombinant technology.
80 Microplasmin does not bind to fibrin and is inhibited by α
2-antiplasmin 100 times slower than is plasmin.
81 Microplasmin showed rapid thrombolysis but no clinical benefit in patients with peripheral arterial or graft occlusion,
82 and trials in ischemic stroke have been curtailed after a phase II study
83; studies in vitreoretinal disease have shown positive results.
84 Delta-plasmin is a recombinant molecule composed of plasmin kringle 1 spliced to the serine protease domain; it retains the same fibrin-binding capacity and fibrinolytic effect as plasmin and is likewise rapidly inhibited by α
2-antiplasmin.
85 Delta-plasmin has a similar safety profile as full-length plasmin in a preclinical model of bleeding
86 but has not yet been assessed in clinical trials.
A recombinant derivative of fibrolase (alfimeprase), isolated from the venom of the Southern copperhead snake, degrades fibrinogen and fibrin and is inhibited relatively slowly by α
2-macroglublin.
87 Alfimeprase showed promise in an early trial
88 in patients with peripheral arterial or graft occlusion, but results from a subsequent study were not better than placebo, and release of bradykinin from low molecular weight kininogen caused hypotension in some recipients.
89 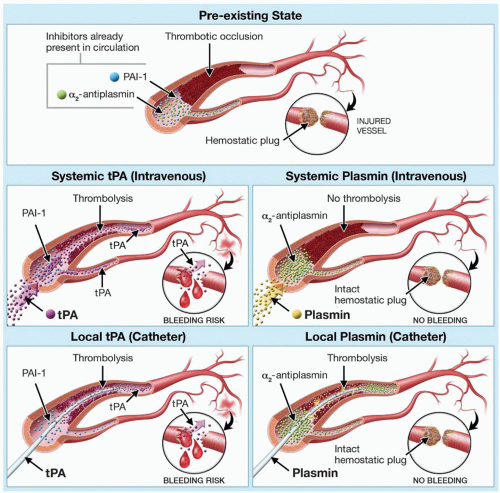


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access