Chapter 19 Fosamprenavir and Amprenavir
INTRODUCTION
Protease inhibitors (PIs) are one of the mainstays of HIV/AIDS therapy,1 being largely responsible for the extraordinary health status improvements witnessed during the last decade in HIV-infected persons who had access to therapy.2,3 The introduction of PIs transformed the natural history of HIV infection. Fifteen years after the description of the first AIDS cases,4–6 PI-based therapy was the first strategy able to slow down and even reverse HIV infection progression toward severe co-morbidity, functional impairment and death. The advent of PIs in combination with two nucleoside-analog reverse transcriptase inhibitors (NRTI) led to the coining of the term “highly active antiretroviral therapy” (HAART)7,8; the concept that would later incorporate triple drug therapies based on non-nucleoside reverse transcriptase inhibitors (NNRTIs),9,10 as well. The first generation of PIs exerted an unprecedented antiviral efficacy in combination with other drugs, being unambiguously clear that early PIs saved thousands of lives.11,12 It is estimated that the increase in the use of lamivudine, indinavir, saquinavir, and ritonavir, drugs approved by the FDA in late 1995 and early 1996, was responsible for more than 90% of the drop in mortality rates from 1995 to 1998.11 In addition, initial PI-based therapy was clearly a cost-effective strategy.12 However, first generation PIs had four important limitations; a very high pill burden, frequent and sometimes severe or life-threatening toxicities, significant pharmacokinetic nuisances with multiple drugs, food and fluid interactions, and broad cross-family resistance, which constrained the administration of other PIs in second-line regimens.
Subsequent PI development has intended to overcome these limitations without compromising antiretroviral potency.13 Modern PIs are even more potent than first generation PIs, but have a much more favorable adherence, tolerability, pharmacokinetic and resistance profiles. The strategy of boosting PI plasma levels with small doses of ritonavir has enabled further pharmacokinetic improvements such as once- or twice-daily administration, avoidance of food interactions and reduced pill dosing. Ritonavir boosting confers a significant enhancement to the PI resistance barrier as well. This is of clear clinical utility in first-line and salvage regimens alike, because it helps prevent the emergence of resistance and surpass acquired decreases in susceptibility. In addition, some modern PIs have particular resistance pathways, different from those of other drugs in the family. For instance, D30N mutation is characteristically selected during early nelfinavir failure in subjects infected with subtype B viruses.14 In those with non-B viruses the first mutation is L90M.15,16 Early atazanavir failure is due to the I50L mutation,17 which decreases the susceptibility to atazanavir but is associated with hypersusceptibility to all other PIs. Similarly, the signature resistance mutation associated with amprenavir and fosamprenavir is I50V.18,19 Early detection of virological failure with these PIs may permit the sequential use of other PIs in subsequent treatment lines, and this effectively widens the therapeutic arsenal against HIV infection.
Amprenavir (APV, Agenerase GlaxoSmithKline) is a PI with demonstrated antiviral efficacy and generally good tolerability when given in combination with other antiretroviral agents. It is a PI with a demonstrated antiviral efficacy and generally good tolerability when given in combination with other antiretroviral agents.20 It also has a particular resistance profile,20 which is different from other PIs and facilitates salvage therapy with other PIs if virological failure is detected before multiple resistance mutations accumulate. However, amprenavir has low water solubility and high lipophilicity, which results in highly variable oral bioavailability21 and requirement of a large proportion of organic excipients to aid in gastric dissolution.21 This, in turn, mandates a high pill burden (10 pills/day in ritonavir-boosted regimens, 16 in unboosted regimens),22 which complicates adequate adherence and limits convenience.
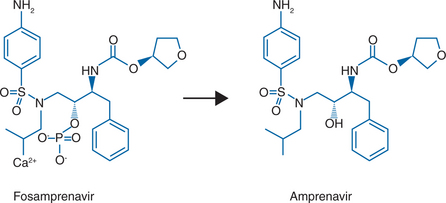
Fosamprenavir (FPV, 908, Lexiva in the United States, Telzir in Canada and the European Union, GlaxoSmithKline and Vertex Pharmaceuticals)18 is the calcium phosphate ester prodrug of amprenavir (Agenerase, GlaxoSmithKline) (Fig. 19-1). Fosamprenavir was synthesized to increase the oral bioavailability of amprenavir, thus allowing a reduction in the pill burden and offering the potential for improved patient compliance. Fosamprenavir has several advantages over amprenavir, including a flexible qd dosing with ritonavir or bid dosing with or without ritonavir, a favorable tolerability profile, a low pill burden, absence of food or fluid restrictions, a particular resistance profile similar to amprenavir’s and distinct from the other PIs, with I50V as the signature mutation, and demonstrated clinical efficacy in antiretroviral-naive subjects. Consequently, fosamprenavir has displaced amprenavir for the treatment of HIV-infected adults whereas amprenavir is still the treatment of choice in children. In 2005, the manufacturer of amprenavir ceased production of 150 mg capsules for adult use while the 50 mg pediatric capsule and the oral solution are still being manufactured. Studies of fosamprenavir in children are ongoing. It seems legitimate to predict that fosamprenavir will also displace amprenavir in the near future in the pediatric setting. Therefore, for the sake of clinical usefulness and durability of concepts discussed below, this chapter will focus mainly on fosamprenavir, highlighting the different aspects particular to amprenavir with respect to its prodrug, when necessary.
PHARMACOLOGY
Molecular Structure
Fosamprenavir calcium (Lexiva, Telzir) is a prodrug of the PI amprenavir (Agenerase). The chemical name of fosamprenavir calcium is (3S)-tetrahydrofuran-3-yl (1S,2R)-3-[[(4-aminophenyl) sulfonyl](isobutyl)amino]-1-benzyl-2-(phosphonooxy) propylcarbamate monocalcium salt. Fosamprenavir calcium is a single stereoisomer with the (3S)(1S,2R) configuration. The molecular formula is C25H34CaN3O9PS and has a molecular weight of 623.7. The chemical name of amprenavir is (3S)-tetrahydro-3-furylN-[(1S,2R)-3-(4-amino-Nisobutylbenzenesulfonamido)-1-benzyl-2-hydroxypropyl] carbamate. Amprenavir is a single stereoisomer with the (3S)(1S,2R) configuration. Its molecular formula is C25H35N3O6S and has a molecular weight of 505.64. The structural formula of fosamprenavir and amprenavir is presented in Figure 19.1. Fosamprenavir and amprenavir are white to cream-colored solids with a solubility of ∼0.31 and 0.04 mg/mL in water at 25°C, respectively.
Drug Forms
Fosamprenavir is commercialized in film-coated tablets for oral administration in strength of 700 mg of fosamprenavir calcium, which is equivalent to amprenavir 600 mg. Amprenavir is commercialized in two oral formulations for pediatric use: 50 mg capsules and oral solution. In 2005, amprenavir’s manufacturer ceased the production of the 150 mg capsules for adult use. Amprenavir oral solution presents amprenavir solubilized with propylene glycol (Table 19-1),18 which is a key dose-limiting factor of this presentation. The recommended daily dose of amprenavir oral solution is 22.5 mg/kg twice daily. This dose corresponds to a propylene glycol intake of 1650 mg kg−1 day−1. Propylene glycol is a viscous, colorless liquid solvent used with many drugs with poor aqueous solubility.23 Acceptable intake of propylene glycol for pharmaceuticals has not been established but it is known that high doses are toxic and may be especially harmful in children of age four or younger. Formerly considered safe, propylene glycol has been associated with hyperosmolality, anion gap metabolic acidosis, hemolysis, osmol gap, central nervous system depression, arrhythmias,1,24–33 and, although less common, renal dysfunction.34–37
Table 19-1 Inactive Ingredients included in Fosamprenavir and Amprenavir Presentations
| Presentation | Inactive Ingredients |
|---|---|
| Fosamprenavir (per each 700 mg tablet) | Colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and povidone K30. The tablet film coating contains hypromellose, iron oxide red, titanium dioxide, and triacetin. |
| Amprenavir 50 mg capsules (per each 50 mg capsule) | d-alpha tocopheryl polyethylene glycol 1000 succinate (TPGS), polyethylene glycol 400 246.7 mg, and propylene glycol 19 mg. The capsule shell contains the inactive ingredients d-sorbitol and sorbitans solution, gelatin, glycerin, and titanium dioxide. The soft gelatin capsules are printed with edible red ink. Each 50-mg amprenavir capsules contains 36.3 IU vitamin E in the form of TPGS. The total amount of vitamin E in the recommended daily adult dose of 50 mg amprenavir capsules is 1744 IU. |
| Amprenavir oral solution (per 1 mL) | Acesulfame potassium, artificial grape bubblegum flavor, citric acid (anhydrous) d-alpha tocopheryl polyethylene glycol 1000 succinate (TPGS), menthol, natural peppermint flavor, polyethylene glycol 400 (170 mg), propylene glycol (550 mg), saccharin sodium, sodium chloride, and sodium citrate (dihydrate). Each mL of amprenavir oral solution contains 46 IU vitamin E in the form of TPGS. |
Adapted from GlaxoSmithKline. Lexiva (fosamprenavir calcium) tablets: prescribing information (US). Online. Available: http://us.gsk.com/products/assets/us_lexiva.pdf. 10 Feb 2006.
Mechanisms of Action
Amprenavir is a sulfonamide that inhibits the postranscriptional processing of Gag and Gag-Pol protein precursors, thereby arresting maturation of nascent virions and blocking their infectivity.38–40 Amprenavir was specifically designed for anti-HIV activity and to enhance binding to the aspartate residues of the HIV-1 protease enzyme,41 based on homology with cellular aspartyl protease22,42 and with the aim of enhancing efficacy on viruses resistant to other PIs.22,42 Amprenavir binds very specifically to HIV-1 and HIV-2 proteases. The inhibition constant, KI, is 0.0003 μg/mL (0.6 nM) for HIV-1 and 0.0096 μg/μL (19 nM) for HIV-2 in acutely and chronically infected cells,20,43 whereas it is higher than 0.885 μg/mL (1750 nmol/L) for human proteases.20,43 Like other PIs, amprenavir is inactive or only weakly active against human aspartyl proteases such as renin, pepsin, and cathepsin.38–40
In Vitro Activity
Fosamprenavir has virtually no antiviral activity in vitro. The in vitro antiviral activity observed with fosamprenavir is due to the presence of trace amounts of amprenavir.18 Amprenavir has a relatively high protease-binding affinity compared to other members of its class because it is more compact and has a molecular backbone that may minimize the intermolecular strain upon binding with the HIV protease. This favors tighter, more specific interaction with the catalytic aspartic acid residues and enzyme flaps. Amprenavir is highly lipophilic and rapidly accumulates within CD4+ T lymphocytes in a concentration-dependent manner. Intracellular concentrations are four times higher than extracellular concentrations, which is attributable to cytosolic protein binding. Amprenavir inhibits the HIV-1 and HIV-2 proteases competitively, with KI values of 0.60 and 19 nM, respectively. The compound is highly selective (>5000-fold) for HIV protease versus human aspartic proteases such as renin, pepsin, and cathepsin, which have KI values of 1750 nM, 3200 nM, and more than 10 000 nM, respectively; and it has low median cytotoxicity (TC50 > 50 μM) across a wide panel of human cell lines.
Amprenavir has a potent in vitro antiviral activity, as evaluated against HIV-1 IIIB in both acutely and chronically infected lymphoblastic cell lines (MT-4, CEM-CCRF, H9) and in peripheral blood lymphocytes. The 50% inhibitory concentration (IC50) of amprenavir ranged from 0.012 to 0.08 μM in acutely infected cells and was 0.41 μM in chronically infected cells (1 μM = 0.50 mcg/mL). Several experiments indicate that protein binding should not significantly alter the antiviral activity of amprenavir.44 The projected minimum drug concentration, corrected for plasma protein binding, exceeded the IC90 of wild type HIV-1 by at least 15-fold.45In vitro, amprenavir exhibits synergistic anti-HIV-1 activity with abacavir, didanosine, zidovudine saquinavir, and additive anti-HIV-1 activity in combination with indinavir, nelfinavir, and ritonavir.18
Amprenavir inhibits Giardia lamblia at 5 μg/mL. No activity has been demonstrated against other protozoans, fungi, or bacteria, herpes simplex viruses 1 and 2, varicella-zoster virus, human cytomegalovirus, coronavirus, yellow fever virus, respiratory syncytial virus, rotavirus, influenza A virus, or rhinovirus at concentrations up to and including 100 μM. In addition, amprenavir showed no activity against human coronavirus protease or in a human papillomavirus DNA replication assay.18,44
Pharmacokinetics
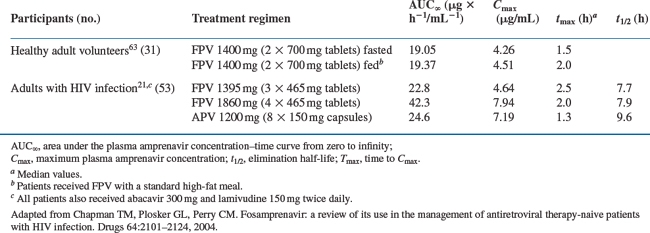
Fosamprenavir is quickly hydrolyzed by hydrolases in intestinal epithelial cells (presumably by alkaline phosphatase46) into its active metabolite, amprenavir, plus an inorganic phosphate.41 Amprenavir is rapidly absorbed into the blood with virtually no fosamprenavir available systemically thereafter.41 Preclinical studies suggest that fosamprenavir is completely converted to amprenavir and is not absorbed.47 Understandably, the pharmacokinetic properties of fosamprenavir and amprenavir are identical beyond this point. Both fosamprenavir and amprenavir are usually given with small doses of ritonavir. This factor modifies the pharmacokinetics of the active moiety amprenavir (Table 19-2), permits higher trough levels (which are associated with increased suppression of viral replication), lower peak levels (which reduces toxicity), and much lower pill burden and less frequent dosing intervals (which improves adherence).
Table 19-2 Mean Pharmacokinetic Parameters of Amprenavir after Single-dose Administration of Fosamprenavir (FPV) or Amprenavir (APV) to Healthy Adult Volunteers or Adults with HIV Infection
Absorption
The median Tmax of amprenavir, given as fosamprenavir, is 2.5 h (ranging between 1.5 and 4 h) whereas, when given as amprenavir, the Tmax is between 1 and 2 h. The absorption of fosamprenavir is not affected by food. Conversely, amprenavir may be taken with or without food, but not with high-fat meals because high fat intake decreases the absorption of this drug moderately. The absolute bioavailability of amprenavir after administration of fosamprenavir has not been established.18 The bioavailability and t½ of amprenavir appears to be highly variable between patients (30–90% and 2–10 h, respectively).41 When amprenavir is combined with ritonavir, the interindividual variations are decreased.19 However, the relative bioavailability of amprenavir oral solution is 14% lower than that of the capsule presentation. Therefore, the recommended dose of the oral solution per body mass index is 11% higher than that for capsules.
Distribution
The first pass of amprenavir is less important than with other PIs.19 After administration of amprenavir, the apparent volume of distribution of the drug is ∼430 L in healthy adults. About 90% of amprenavir is bound to plasma proteins, primarily to α1-acid glycoprotein. Although albumin concentrations are relatively stable among individuals, variations in α1-acid glycoprotein concentrations may fluctuate widely and are a significant predictor of amprenavir apparent total clearance.48 It has been suggested that differences seen in amprenavir pharmacokinetics could be due to variable α1-acid glycoprotein concentrations in vivo. However, α1-acid increases in glycoprotein concentration could also affect the intracellular concentration of PIs and, consequently, their antiviral efficacy.49 The unbound fraction of amprenavir remains unchanged when ritonavir is added. Concentration-dependent binding was seen in vitro over the concentration range of 1–10 mcg/mL, with decreased binding at higher concentrations. As commented above, several experiments indicate that protein binding does not significantly alter the antiviral activity of amprenavir.44
Metabolism
Fosamprenavir is rapidly and almost completely hydrolyzed to amprenavir and inorganic phosphate in the gut epithelium, during absorption and before reaching the systemic circulation. Amprenavir is metabolized in the liver by the cytochrome P450 3A4 (CYP3A4) enzyme system and, to a lesser extent, by CYP2D6, CYP2C9, and CYP2C19,44 resulting in two major metabolites from oxidation of the tetrahydrofuran and aniline moieties. Glucuronide conjugates of oxidized metabolites can be seen as minor metabolites in urine and feces. Approximately 75% and 14% of a single 14C-labeled amprenavir dose was detected as metabolites in feces and urine, respectively.43
Elimination
The plasma elimination half-life of amprenavir is ∼7.7 h.18 Amprenavir is mostly eliminated in the feces after being metabolized into two major metabolites. Virtually no unchanged amprenavir is seen in feces. Less than 1% of the administered dose is seen in urine as unchanged amprenavir.
Pharmacokinetics in Special Populations
The pharmacokinetics of amprenavir after the administration of fosamprenavir or amprenavir have only been studied in asymptomatic adult HIV-infected patients and in healthy volunteers. Contrary to amprenavir, fosamprenavir has not been studied in patients with hepatic insufficiency. After the administration of 600 mg of amprenavir, the drugs AUC was greater in patients with moderate and severe cirrhosis compared to healthy volunteers. No pharmacokinetic differences have been observed according to gender or race in either fosamprenavir or amprenavir presentations. It is important to note that amprenavir pharmacokinetics have not been studied in patients with renal insufficiency or in people older than 65 years of age. Due to its practically nil renal elimination, this should not be a major limitation for administering fosamprenavir or amprenavir in patients with renal failure. Conversely, caution is recommended if these drugs need to be administered in elder patients.
Pharmacokinetics of fosamprenavir have not been studied in children. However, amprenavir pharmacokinetics are being investigated in the pediatric population. A phase I, open label, dose-escalating trial in 20 HIV-infected children 4 to 12 years of age,50 evaluated four amprenavir doses of 5, 10, 15, and 20 mg/kg of body weight, administered as soft gelatin capsules. Amprenavir was rapidly absorbed, with a mean time to maximum concentration (Tmax) occurring 0.95 to 1.58 h after dosing. The area under the concentration-time curve (AUC0→∞) was dose proportional, and the mean maximum plasma concentration (Cmax) increased linearly in a less than dose-proportional manner. Amprenavir was eliminated relatively slowly, with a mean terminal-phase half-life (t½) of 6.17–8.28 h. The t½, apparent total clearance and apparent volume of distribution during the elimination phase were dose independent. Considerable interpatient variability was seen for all pharmacokinetic parameters of amprenavir. The results of this study suggested that 20 mg/kg of amprenavir administered twice a day should be used in future pediatric studies. Of note, amprenavir capsules and oral solution are not interchangeable on a milligram-per-milligram basis. Also, oral solution is contraindicated in children below the age of 4 years because of the potential toxic effects of the excipient propylene glycol.18
Drug Interactions
Amprenavir is metabolized by, inhibits, and may induce the CYP3A4 pathway. Both fosamprenavir and amprenavir have the potential to interact with other drugs that are inducers, substrates or inhibitors of this enzyme system.43,18 In clinical practice, fosamprenavir is usually co-administered with small doses of ritonavir with the purpose of boosting amprenavir plasma concentrations. Even at these small doses, ritonavir is a potent inhibitor of the cytochrome P450 enzyme complex, thus playing an additional role in drug–drug interactions seen with fosamprenavir. In patients receiving ritonavir-boosted fosamprenavir, it is advisable to consider ritonavir’s particular interaction profile in addition to that of amprenavir.
Interactions with other PIs
Interactions with NNRTIs
Interactions with CCR5 Inhibitors
Fosamprenavir/ritonavir has no significant effect on the pharmacokinetics of vicriviroc/ritonavir. Plasma AUC and Cmax of the CCR5 inhibitor vicriviroc, are comparable if this drug is given with ritonavir (100 mg bid) or with fosamprenavir/ritonavir (700 mg/100 mg bid).56 Apparently, neither modification to vicriviroc dosing, nor TDM is needed when vicriviroc is added to a ritonavir-boosted fosamprenavir regimen. No information is yet available for other CCR5 inhibitors, nor for CXCR4 inhibitors.
Interactions with Non-Antiretrovirals
Most interactions with non-antiretroviral drugs have been described with amprenavir. Since fosamprenavir is quickly metabolized to amprenavir by intestinal hydrolases and then absorbed as amprenavir, it is assumed that virtually all interactions are common to both formulations. Drugs that reduce the intestinal absorption of amprenavir by altering the intestinal pH, like the antacids, or metabolic inducers like carbamazepine or phenytoin, reduce amprenavir plasma levels, leading to a decreased antiviral efficacy of this drug. Inhibitors of amprenavir’s metabolism, like the CYP450 inhibitors, increase amprenavir plasma levels, therefore increasing its toxicity. On the other hand, amprenavir’s inhibition of CYP450 increases plasma drug levels of benzodiazepines, psychoactive drugs, antiarrythmics, antihypertensives, prokinetics, antihistamines, HMG CoA reductase inhibitors, certain antibacterials, antimycobacterials, oral contraceptives and oral anticoagulants. This leads to an increase in frequency and intensity of their usual adverse events. In practice, fosamprenavir is usually co-administered with ritonavir. Therefore, additional interactions of ritonavir with other non-antiretroviral compounds must be expected, as well. Clinically significant drug interactions with amprenavir are summarized in Table 19-3.
Table 19-3 Clinically relevant Interactions of Fosamprenavir with Non-antiretroviral Drugs
| Family | Drugs | Reason |
|---|---|---|
| Drugs that are contraindicated with Fosamprenavira | ||
| Antiarrhythmics | Flecainide or Propafenoneb | Risk of serious and life-threatening reactions (e.g., cardiac arrhythmias). |
| Ergot derivatives | Dihydroergotamine, Ergonovine, Ergotamine, Methylergonovine | Risk of serious and life-threatening reactions (e.g., ergot toxicity with peripheral vasospasm and acral ischemia). |
| Neuroleptics | Pimozide | Risk of serious and life-threatening reactions (e.g., cardiac arrhythmias). |
| Prokinetics | Cisapride | Risk of serious and life-threatening reactions (e.g., cardiac arrhythmias). |
| Benzodiazepines | Midazolam or Triazolam | Risk of serious and life-threatening reactions (e.g., prolonged or increased sedation or respiratory depression). |
| Drugs that should not be co-administered with Fosamprenavir | ||
| Antimycobacterials | Rifampin | Reduces amprenavir plasma concentrations by 90% when administered with nonboosted fosamprenavir. Unknown pharmacokinetics when administered with ritonavir-boosted fosamprenavir. |
| HMG-CoA reductase inhibitors | Lovastatin and Simvastatin | Highly dependent on CYP3A4-mediated elimination. Amprenavir-mediated CYP3A4 inhibition increases risk of rhabdomyolysis and toxic myopathy. |
| Herbal products | St John’s Wort (Hypericum perforatum) | Significant reduction of amprenavir plasma concentrations, leading to suboptimal drug levels, virological failure and drug resistance. |
| Drugs that require dose reduction when co-administered with Fosamprenavir | ||
| HMG-CoA reductase inhibitors | Atorvastatin | Also dependent on CYP3A4-mediated elimination, but less than lovastatin and simvastatin. Use ≤20 mg/day of atorvastatin with careful monitoring or consider other HMG-CoA reductase inhibitors such as fluvastatin, pravastatin or rosuvastatin. |
| Benzodiazepines | Alprazolam, Clorazepate, Diazepam, Flurazepam | Increase in benzodiazepine levels with unknown clinical significance. However, benzodiazepine dose reduction may be needed according to clinical tolerance. |
| Antimycobacterials | Rifabutin | Frequent blood count monitoring is warranted to detect neutropenia. Reduce dose of rifabutin by 50% if given with nonboosted fosamprenavir and by 75% (with a maximum dose of 150 mg every other day or three times weekly) if given with fosamprenavir/ritonavir. |
| PDE5 inhibitors | Sildenafil, Vardenafil | Increases sildenafil and vardenafil plasma levels increasing the risk of side effects, such as hypotension, blurry vision, priapism 4 h after intake. Sildenafil should be used at 25 mg every 48 h with adverse event monitoring. Vardenafil should be used with caution at doses no more than 2.5 mg every 24 or 72 h if fosamprenavir is given without or with ritonavir, respectively. |
| Azole antifungals | Itraconazole or Ketoconazole | Increase monitoring for adverse events due to ketoconazole or itraconazole. In patients treated with nonboosted fosamprenavir, dose reduction of ketoconazole or itraconazole below 400 mg/day may be needed. In patients treated with fosamprenavir/ritonavir, avoid doses of ketoconazole or itraconazole <200 mg/day. |
| Drugs that require dose increase when co-administered with Fosamprenavir | ||
| Opioids | Methadone | Co-administration of amprenavir and methadone, decreases methadone plasma levels as well as decreases by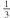 amprenavir plasma, levels as compared with a historical cohort. amprenavir plasma, levels as compared with a historical cohort. |
| Drugs that require plasma concentration monitoring if administered with fosamprenavir | ||
| Antiarrhythmics | Amiodarone, Lidocaine (systemic), Quinidine | Risk of cardiac arrhythmias. |
| Oral anticoagulants | Warfarin | Risk of bleeding. Requires close INR monitoring. |
| Tricyclic antidepressants | Amitriptyline, Amoxapine, Clomipramine, Desipramine, Doxepin, Imipramine, Nortriptyline, Protriptyline, Trimipramine | Risk of serious side-effects. |
| Immunosuppressants | Cyclosporine, Sirolimus, Tacrolimus | Significant interactions with narrow therapeutic index. Plasma level monitoring is warranted to avoid toxicity or suboptimal immune suppression. |
| Other drugs with significant interactions with Fosamprenavir | ||
| Antacids | May decrease amprenavir absorption. Antacids should be taken at least one hour before or after amprenavir. This interaction has not been evaluated with ritonavir-boosted fosamprenavir. | |
| Calcium channel blockers | Diltiazem, Felodipine, Nifedipine, Nicardipine, Nimodipine, Verapamil, Amlodipine, Nisoldipine, Isradipine. | Risk of hypotension. Caution is warranted and clinical monitoring of patients is recommended. |
| Antiarrhythmic | Bepridil | Use with caution. Increased bepridil exposure may be associated with lfe-threatening reactions such as cardiac arrhythmias. |
| Psycoactive compounds | Trazodone | Use with caution. Dose reduction may be required. |
| Anticonvulsants | Carbamazepine, Phenobarbital, Phenytoin | Induce CYP3A4 and reduce amprenavir plasma concentrations, leading to suboptimal drug levels, virological failure and drug resistance. |
| Corticosteroids | Dexamethasone | Induces CYP3A4 and reduces amprenavir plasma concentrations. |
| Fluticasone | Risk of accumulation and adverse effects. Use alternative drugs if prolonged fosamprenavir use is expected. | |
| Oral contraceptives | Ethinyl estradiol/norethindrone | Co-administration with fosamprenavir alters hormonal levels. |
| Alternative methods of birth control are recommended. | ||
Based on prescribing information for LEXIVA fosamprenavir calcium.18
a These drugs are highly dependent on CYP3A4 for clearance, have a narrow therapeutic index, or high plasma concentrations are associated with serious and life-threatening effects.
b Flecainide and propafenone are contraindicated if fosamprenavir is co-administered with ritonavir. If fosamprenavir is administered without ritonavir, plasma concentration monitoring is warranted.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree