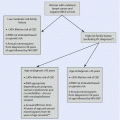
Degree of risk of permanent amenorrhoea
Age (years)
Cancer treatment regimen for breast cancer
High risk (>80%)
≥40
CMF, CEF,CAF,TAC x 6 cycles
Moderate risk (40–60%)
≥40
30–39
any
AC x 4 cycles
CMF, CEF,CAF,TAC x 6 cycles
AC or EC x 4 → Taxanes
Low risk (<20%)
≤40
≤30
AC x 4 cycles
CMF, CEF,CAF,TAC x 6 cycles
Very low or no risk
Any
Methotrexate
Fluorouracil
Although still controversial, several studies have reported the favourable effects of chemotherapy-induced amenorrhoea on breast cancer outcomes [9–12]. However as more women survive their cancers, more are confronted with the long-term side effects of treatment, including infertility.
Though more than half of young women treated for breast cancer may desire a future pregnancy [13], these patients have the lowest pregnancy rate among cancer survivors, with an overall 67% reduction in the chance of a future pregnancy as compared to the general population of the same age [4]. This may be due to the gonadotoxic effect of chemotherapy, prolonged treatment with tamoxifen, and/or fear of disease recurrence causing reticence to become pregnant. Besides infertility, POF can cause a significant impact on a patients’ quality of life, particularly from vasomotor symptoms, sleep and mood disturbance, sexual dysfunction, and bone density loss [14].
Even after completion of treatment, these women may not be able to conceive immediately. Timing to ensure a safe pregnancy is as important as concerns for future fertility after cancer treatment. However, the optimal time interval from treatment completion to pregnancy is still unknown. The risk of mutagenesis is maximal during the maturation phase of the oocyte, which is approximately 6 months [15]. As most cytotoxic agents are mutagenic and teratogenic to oocytes, it is common practice to delay conception by a minimum of 6 months after completing treatment. Thus, the timing of pregnancy should take into consideration the duration of treatment and time from completion, the patient’s age and ovarian function, and the risk of relapse [4].
After completion of primary systemic or adjuvant treatments, some women may not be able to conceive naturally and assisted fertility techniques may be required. However, there is only one small retrospective study available that looked at the feasibility and safety of performing assisted reproductive technology in patients who had already been treated with chemotherapy [16]. The study suggested that this approach could be considered in selected patients who cannot conceive spontaneously after completion of adjuvant therapy and who did not undergo fertility preservation techniques at diagnosis [16]. Nevertheless, more data are needed to confirm whether assisted reproductive technology after anticancer treatments is safe in breast cancer survivors.
43.3 Fertility Preservation Techniques
The American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), the European Society of Medical Oncology (ESMO), and the International Consensus Conference for Breast Cancer in Young Women recommend reproductive counselling, ideally before commencing any anticancer treatment [3–6].
Fertility preservation techniques may either use surgical or medical strategies. These include embryo cryopreservation, oocyte cryopreservation, ovarian tissue cryopreservation, or ovarian suppression with gonadotropin-releasing hormone (GnRH) agonists. The success of each method is strongly dependent on the patient’s ovarian reserve. Notably, the published success data on these techniques are widely based on infertile women rather than on cancer survivors. More importantly, resumption of menses does not necessarily translate to successful fertility outcomes, i.e. pregnancy and live birth. ◘ Table 43.2 summarizes the different fertility preservation strategies and outcomes.
- 1.
Embryo cryopreservation and mature oocyte cryopreservation
Both embryo cryopreservation and oocyte cryopreservation are the standard recommended fertility preservation strategies in female cancer patients, though the acceptability and availability vary in certain countries. Vitrification (ultrarapid freezing) appears to be superior to a slow freezing method [24], and the success rate is age- (<38 years) and centre-dependent. Embryo cryopreservation needs a sperm donor for embryo creation, and therefore only those women with steady partner will be able to undergo embryo cryopreservation.
Both strategies require at least 2 weeks of hormonal manipulation (i.e. controlled ovarian stimulation) to allow the collection of the oocytes to be then cryopreserved or fertilized (i.e. embryos) before cryopreservation. Standard protocols for controlled ovarian stimulation last about 9–15 days, starting at the onset of menses. Hence, two main issues should be considered in cancer patients: a possible 2–6 weeks delay in the initiation of chemotherapy and the temporary increase in oestradiol levels with a potentially negative impact on the prognosis of patients, especially in those with hormone receptor-positive breast cancer [7].
Two strategies have been developed to address these issues in breast cancer patients, including the use of «random-start protocols» [25] to avoid delay in treatment initiation or use of controlled ovarian stimulation with tamoxifen or letrozole [26, 27] to reduce the exposure risk to high oestradiol levels during ovarian stimulation.
«Random-start protocols» allow ovarian stimulation to start at any time during the menstrual cycle, even in luteal or late follicular phases, and experience has shown promising results in terms of successful oocyte collection [25].
In breast cancer patients, particularly in those with hormone receptor-positive disease, ovarian stimulation raises the added concern of inducing a sustained hyper-oestrogenic state, with potential acceleration of tumour growth and risk of cancer recurrence. Gonadotropin stimulation combined with endocrine agents (i.e. tamoxifen or letrozole) has led to successful fertility preservation while maintaining oestrogen levels within the physiologic range [26, 27]. Furthermore, no observed negative consequences on the quality of oocytes and embryos collected and cryopreserved were observed with the use of these protocols [26, 27]. The only prospective single-centre study investigating the long-term safety of controlled ovarian stimulation with letrozole supplementation (COSTLES) in breast cancer patients showed reassuring results [28]. After 5 years of median follow-up, no significant difference in the relapse-free survival was observed between patients who underwent embryo cryopreservation and those who did not (hazard ratio [HR] = 0.77; p = 0.61) [28]. On subgroup analysis, no significant difference in relapse-free survival was observed in both patients with hormone receptor-positive and hormone receptor-negative disease and in both patients with BRCA mutations and those without mutations between those undergoing ovarian stimulation and not [28]. However, longer follow-up and more cases are needed to confirm these findings.
Recently, the same group reported for the first time the success rate of the procedure in terms of fertility preservation in breast cancer patients [19]. The authors reported an overall live birth rate per embryo transfer similar to that of the infertile population without cancer of a similar age (45.0 vs. 38.2; p = 0.2) [19]. Out of 33 patients attempting pregnancy, 17 had at least one child resulting in a fertility preservation rate of 51.5% per attempting woman (◘ Table 43.2) [19].
- 2.
Ovarian tissue cryopreservation
Ovarian tissue cryopreservation is an experimental fertility preservation technique that requires two surgical procedures – first, for removal of the cortical ovarian tissue and, second, for future reimplantation into the pelvic cavity (orthotopic site) or outside the peritoneal cavity (heterotopic site), commonly in the forearm [29].
This technique offers several advantages including rapid restoration of ovarian function (within 3–6 months) after successful transplantation and minimal delay in the initiation of anticancer treatment after surgery. However, malignant cell contamination is one potential implication, though the risk is deemed low in breast cancer, except for those with a known BRCA gene mutation [30] where the development of ovarian cancer poses a significant risk.
A successful recovery of ovarian function is expected in the majority of patients, with possible sustained longevity of function [31]. While successful pregnancy has been reported with this strategy, live birth rates have been variable and mostly reliant on less robust methodological studies, with an estimated pregnancy rate of approximately 25% (◘ Table 43.2) [21].
- 3.
Ovarian suppression with GnRH agonists during chemotherapy
The ovaries may potentially be protected during chemotherapy by putting them into a dormant state. This may be achieved by hormonal manipulation using gonadotropins, although there are concerns. Concurrent use of GnRH agonists with chemotherapy is a promising strategy as it is easily accessible in terms of cost and timing, well tolerated in terms of short-term side effects (i.e. vasomotor symptoms), and therefore may be a good alternative strategy when access to embryo cryopreservation or oocyte cryopreservation is not feasible.
The concerns for concurrent GnRH agonist use are mainly attributed to the lack of chemotherapy-induced amenorrhoea having a possible negative prognostic effect on survival, the potential negative interactions with chemotherapy, and the initial flare-up effect causing a transient hyper-oestrogenic state, possibly increasing the risk of breast cancer recurrence, particularly in hormone receptor-positive tumours [32].
Previous trials evaluating the role of ovarian suppression during breast cancer chemotherapy have reported conflicting results on the efficacy of the procedure in terms of reducing the risk of developing POF after chemotherapy at short follow-up (6–36 months), while limited data are available at longer follow-up; hence the practice was considered unreliable [3, 33–35]. However, ovarian suppression with GnRH agonists has recently gained much interest, with two large randomized studies showing favourable results.
The POEMS/S0230 trial randomized 257 premenopausal women (median age of 38 years) with hormone receptor-negative breast cancer to standard chemotherapy with or without goserelin, with a 2-year rate of ovarian failure as the primary endpoint [22]. This was defined as the absence of menses in the preceding 6 months and follicle-stimulating hormone levels in the postmenopausal range. Despite the incomplete data available, adding goserelin to chemotherapy resulted in a significantly lower ovarian failure rate (8% vs. 22%, odds ratio OR = 0.30, 95% confidence interval [CI] 0.09–0.97; p = 0.04), lower 2-year ovarian dysfunction rate (14% vs. 33%, OR = 0.35, 95% CI 0.13–0.93, p = 0.03), higher pregnancy rate (21% vs. 11%, p = 0.03), and superior 4-year disease-free survival (89% vs. 78%, p = 0.04) than chemotherapy alone [22]. Following these positive results, the panel of the St. Gallen Consensus 2015 supported the use of ovarian function suppression with GnRH agonists during chemotherapy to preserve ovarian function and fertility in women with hormone receptor-negative disease [36]. Likewise, the NCCN recommends ovarian suppression with GnRH agonists during adjuvant chemotherapy in premenopausal women with hormone receptor-negative tumours to preserve ovarian function and diminish the risk of chemotherapy-induced amenorrhoea [5].
The PROMISE-GIM6 trial randomized 281 patients with early breast cancer to chemotherapy with or without triptorelin, with the incidence of early menopause as the primary outcome [37]. This was defined as the absence of menstrual activity and follicle-stimulating hormone and oestradiol at postmenopausal levels, 1 year after the last chemotherapy cycle. The trial included patients with both hormone receptor-positive and hormone receptor-negative tumours. Results showed a lesser rate of chemotherapy-induced early menopause (8.9% vs. 25.9%, p < 0.001) in the triptorelin arm [37]. Updated analysis revealed a higher cumulative long-term (5 years) probability of ovarian function recovery with triptorelin than chemotherapy alone (age-adjusted HR = 1.48, p = 0.006) [23]. No statistical difference in the pregnancy rate was observed although more patients receiving ovarian suppression during chemotherapy had a subsequent pregnancy (8 vs. 3; age-adjusted HR = 2.40, p = 0.20) [23]. The strategy did not reveal a negative disease-free survival impact in patients with hormone receptor-positive disease.
The favourable safety and efficacy data from these two consecutive trials have changed the mindset of several experts on recommending ovarian suppression with GnRH agonists during chemotherapy as a reliable strategy for ovarian function and fertility preservation irrespective of hormone receptor status of the tumour [6, 7].
Three meta-analyses of randomized controlled trials on the effect of ovarian suppression during chemotherapy as a strategy to preserve ovarian function and fertility in breast cancer patients were published in 2015. Lambertini and colleagues reported a reduced risk of chemotherapy-induced POF and an increased pregnancy rate, without a negative impact on prognosis [38], while Munhoz and colleagues showed a higher rate of menstrual recovery at 6 and 12 months but not fertility or pregnancy in premenopausal women with early breast cancer when GnRH agonist was given concurrently with chemotherapy [39]. Similarly, Shen and colleagues reported a higher rate of menstrual return without any protective effects on fertility [40]. These findings highlight that resumption of menstruation is still a poor surrogate for fertility and that GnRH agonist use, although very promising, must not be the only method relied on to preserve fertility, especially when access to embryo cryopreservation or oocyte cryopreservation are viable options.
Table 43.2
Fertility preservation strategies and outcomes
Techniques | Procedure
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|