(1)
Department of Pathology Beckley Veterans Medical Center (West Virginia), McGuire Veterans Medical Center, Virginia Commonwealth University, Richmond, VA, USA
Cervix
Review of Pertinent Histology, Physiology
The ectocervix of the adult female is lined by a nonkeratizing squamous epithelium which shows cyclical changes in response to local steroid hormonal levels [1]. ER and PR are present in the epithelium and stromal fibroblasts. The endocervix is covered by a mucinous columnar epithelium which also invaginates to line the cleft-like structures in the stroma (endocervical glands). The glandular clefts are arranged in a collateral tunnellike pattern and usually penetrate less than 5 mm from the surface (but can be as deep as 1 cm). In contrast to the marked cyclic morphological changes in the ectocervical squamous cells, the columnar cells manifest minimal cytological changes. Instead, it shows dramatic variations in the production of secretion during the menstrual cycle. Importantly, mitotic activity is extremely rare in benign nongravid columnar epithelium. The subepithelial microvessel network of the endocervix is better developed than that of the ectocervix and the glandular structures largely shun from larger vessels and nerve bundles [2]. Furthermore, the endocervical stroma has twice as many stromal cells as does the ectocervical counterpart. The stromal cell from the two regions has different immunohistochemical features with the former showing reactivity for SMA and the latter for desmin. Moreover, the normal stroma also contains a dense network of CD34+ fibrocytes which are more predominantly located in the subepithelial and perivascular areas. In deep locations of the stroma, smooth muscle fibers are present and they are usually surrounded by CD34+ stromal cells [3].
The cervix plays important roles in the reproductive function. Not only is it involved in providing a port of entry for the sperm and a chemical barrier to infectious microorganism; the cervix also contributes to the creation of a safe haven for the implanting embryo and subsequently the growing fetus and a passageway for the ripe product of conception. The dramatic changes in the process of remodeling and ripening are effected through steroid hormones and complex epithelial–stromal interactions. It is only during the late phase of pregnancy that the endocervical glands are fully developed and manifest as lobules of tightly packed glands [4–6]. The destruction and rearrangement of collage fibers and other changes in the extracellular matrix apparently provide space for this remarkable glandular expansion. During the subsequent postpartum months, the hyperplastic glands involute gradually reverting to the baseline collateral clefts. Defective involution of some of the hyperplastic glands might result in diffuse laminar glandular hyperplasia.
Key Morphological Feature of Well-Differentiated Squamous Cell Carcinoma
Irregular narrow-based squamous nests with no surface connection
Desmoplasia (haphazard SMA myofibroblasts) and loss of CD34+ fibrocytes (Figs. 6.1 and 6.2)
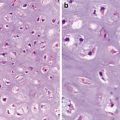
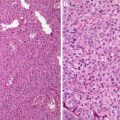
Fig. 6.1
Well-differentiated cervical squamous cell carcinoma. Irregular, narrowly based, or detached squamous nests. Cellular atypia at epithelial–stromal interface
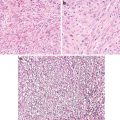
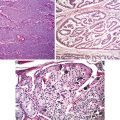
Fig. 6.2
Stroma of cervical squamous cell carcinoma. Note lack of CD34+ fibroblasts (a) in tumor stroma and presence of SMA+ fibroblasts (b). Normal cervical stroma contains plenty of CD34+ fibroblasts (c) and no SMA+ fibroblast (d)
Discussion
Squamous cell carcinoma of the cervix develops from cervical intraepithelial dysplasia with clear HPV involvement. Well-differentiated squamous cell carcinoma is characterized by abundant keratin pearls and individual cell keratinization and a chronically inflamed stroma. Four common criteria used in the evaluation of invasion are (1) desmoplasia, (2) paradoxical maturation (excessive cytoplasmic keratinization with conspicuous nucleoli), (3) loss of cell polarity at the basal layer, and (4) and the blurring of the epithelial–stromal interface.
However, the evaluation of stromal invasion is frequently compromised by tangential sectioning, cautery effect, obscuring inflammation at the tumor border and other inflammatory process, and even glandular involvement by metaplasia or dysplasia. In order to avoid overcalling metaplasia or dysplasia involving the glandular clefts, attention should be directed to the smooth contour of the tumor nests, their cytological features in comparison to those of the surface dysplastic epithelium, and polarity of tumor cells. In difficult cases, immunostainings for CD34 and SMA hold the promise for making the distinction [7, 8]. The stromal cells of invasive squamous carcinoma lack reactivity for CD34. SMA stain lights up haphazardly arranged myofibroblasts (desmoplasia). To differentiate pseudoepitheliomatous hyperplasia from well-differentiated squamous cell carcinoma would require the appreciation of the underlying inflammatory process and shape of the invasive components and their connection to the surface and shape (jagged pointed tips and a broad base connected to the surface). In addition to the panel of p53, MMP-1, and Ki67 which is used in other squamous locations, p16 can be used [9, 10].
Important Squamous Cell Carcinoma Variants
Awareness of the presence of variants would avoid passing them off as benign mimics.
Verrucous carcinoma lacks a central fibrovascular core and koilocytosis. Instead of having an infiltrative growth pattern, the tumor has a characteristic pushing border.
Condylomatous (warty) carcinoma has marked condylomatous changes. It has basal atypia and an infiltrative component at the base.
Papillary squamous (transitional) carcinoma has prominent papillary structures lined by cells with squamous and/or transitional cell differentiation. Interestingly, the transitional cells still maintain their cellular polarity. The invasive component manifests as well-circumscribed nests which are composed of squamous cells which are basal cell-like and resemble high-grade dysplasia.
Differential Diagnosis
Condyloma Acuminatum
Condyloma acuminatum shows squamous papillomatous growth with acanthosis, parakeratosis, and hyperkeratosis. Koilocytotic atypia, multinucleation, and individual cell keratinization are evident. The lack of stromal invasion sets it apart from condylomatous carcinoma.
Immature Squamous Metaplasia, Dysplasia Involving Glands
Squamous metaplasia and dysplasia frequently extend downward to the endocervical glands, and they show cellular atypia and mitotic activities. They however remain connected to the surface and keep the smooth glandular contours.
Placental Site Nodule and Epithelioid Trophoblastic Tumor, Decidual Changes
Placental site nodules are well-circumscribed nests composed of intermediate trophoblasts surrounded by hyaline material. Epithelioid trophoblastic tumor also shows preference for nest formation. The tumor often contains geographic necrosis. In difficult cases, immunostainings for CK18, inhibin, and p16 can help make the distinction since the trophoblast is positive for inhibin and CK18 and negative for p16 [11]. The reverse is true for squamous cell carcinoma cells.
Decidual changes can be confused with well-differentiated squamous cell carcinoma because of the abundant pink cytoplasm and well-defined cellular contours. However, the cells are degenerative in nature and a history of recent pregnancy is evident.
Reparative Changes with Chronic Granulomatous Inflammation and Pseudoepitheliomatous Hyperplasia
Reparative changes and pseudoepitheliomatous hyperplasia associated with chronic inflammatory diseases can be mistaken for invasive squamous carcinoma. The presence of extensive chronic inflammatory infiltrate alerts the pathologist to a possible infectious etiology. The hyperplastic squamous projections are usually connected to the surface with a broad base and have a jagged contour with pointed tips. Special stains for microorganisms and p16 can help confirm a diagnosis.
Key Morphological Features of Cervical Well-Differentiated Adenocarcinoma
Nonlobular, irregular glandular, or villoglandular proliferation
Deep location (Figs. 6.3, 6.4, and 6.5)
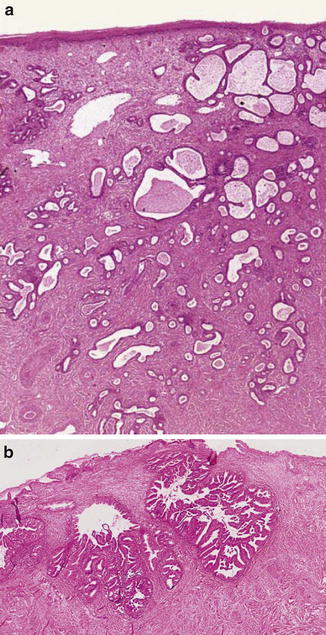
Fig. 6.3
Well-differentiated adenocarcinoma. Nonlobular, irregular glands (a). Expansive growth pattern (b) (Diagnostic Gynecologic and Obstetric Pathology, Elsevier/Saunders, 2011 with permission)
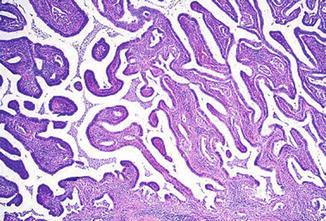
Fig. 6.4
Adenocarcinoma. Villoglandular growth pattern (Diagnostic Gynecologic and Obstetric Pathology, Elsevier/Saunders, 2011 with permission)
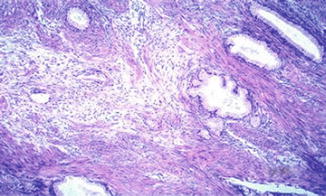
Fig. 6.5
Adenoma malignum. Note bland-looking glands with deep penetration (Diagnostic Gynecologic and Obstetric Pathology, Elsevier/Saunders, 2011 with permission)
Discussion
It has been increasingly realized that there are two pathways for cervical glandular carcinogenesis. The vast majority of endocervical adenocarcinomas are HPV related and progress through the dysplasia to carcinoma in situ progression pathway. The second pathway is independent of HPV infection and follows the pathway from lobular endocervical glandular hyperplasia to minimal deviation adenocarcinoma and mucinous adenocarcinoma [12, 13].
It is generally known that invasion should be suspected if there are nonlobular exuberant glandular growth in the form of extensive budding, confluency, cribriform, and exophytic papillary projections. However, the adenocarcinoma in situ can have complex papillary formation and even cribriform formation, and some benign glandular lesions are not lobular. Deep location, inflammatory, and desmoplasia of the stroma and presence adjacent to large vessels and nerve fibers are some important features of stromal invasion. Nevertheless, they are not always present. For instance, endocervical adenocarcinoma can also invade in expansile pattern without eliciting salient desmoplastic reaction. Some other promising indicators of stromal invasion include increased periglandular expression of SMA-positive myofibroblasts and stromal expression of CD44 and tenascin [14, 15] (Fig. 6.6).
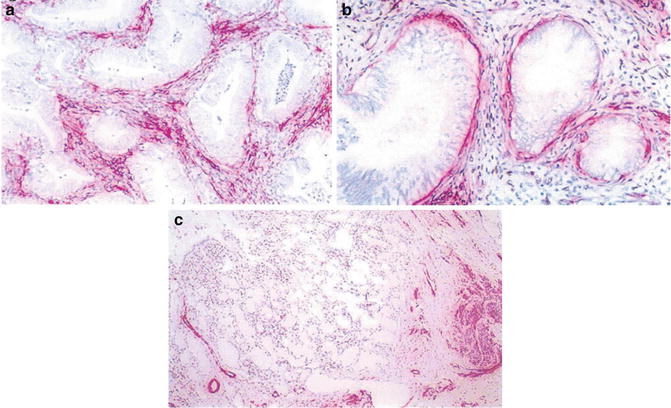

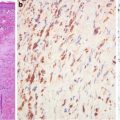
Fig. 6.6
Stroma of adenocarcinoma. Note presence of SMA+ myofibroblasts in stroma (a). A rim of SMA+ myofibroblasts around minimal deviation adenocarcinoma (b). Benign glands between preexisting smooth muscle bundles (c) (Histopathology, John Wiley&Sons, Inc, 2005 with permission)
The depth of glands is a very important index for the minimal deviation adenocarcinoma because it lacks exuberant epithelial growth and contains only focal cytological atypia. Most of the benign glands are confined to the inner 1/3 of the cervical wall. If the questionable glands extend more than 1 mm deeper than the adjacent benign ones, they are considered deep enough. This “deep concept” is not to be confused with those benign lesions usually located in the outer layer of the cervical wall. As discussed in the differential diagnosis section, some benign lesions can also be deep and even transmural.
To differentiate from those benign mimics of adenocarcinoma, a panel of immunostaining can be very helpful. The panel includes CEA, Ki67, and p16. Benign lesions usually show low mitotic activity and are negative for CEA and p16 [16, 17].
Another common dilemma in gynecological pathology is to distinguish a primary cervical adenocarcinoma from uterine extensions since several variants (such as villoglandular, endometrioid, and even mucinous) can be seen in both locations. Clues to a cervical origin include the presence of cervical dysplasia and lack of benign squamous morules and stromal foamy cells. Endocervical adenocarcinoma cells are positive of CEA and negative for ER, PR, and vimentin (the cells at the invasive front can be vimentin positive due to epithelial– mesenchymal transition) [16, 17]. Apparently, the HPV infection messes up the epithelial–stromal coupling through the steroid hormones with the resultant loss of ER and PR expressions [18–20]. Endometrial carcinoma is ER, PR, and vimentin positive and negative for CEA.
Differential diagnosis
Nabothian Cysts and Deep Glands
The lining epithelium of Nabothian cysts is attenuated and sometimes shows squamous metaplasia. When cysts rupture, the spilled mucin can induce reactive changes in both the stroma and the epithelium. However, the cysts lack significant nuclear atypia and an infiltrative pattern even though they might be deep in location.
Deep tubo-endometrioid metaplasia can be mistaken for adenocarcinoma of endometrioid variant. The irregular and crowded glands are composed of columnar cells with high N/C ratios with occasional mitotic figures and are sometimes surrounded by a myxoid to edematous stroma. The presence of cilia and apical snouts are important clues.
Microglandular Hyperplasia
Microglandular hyperplasia consists of tightly packed glands in various sizes and shapes and may involve both the surface and deeper endocervical clefts. In general, the cells are bland with only occasional nuclear atypia. The lesion can be diagnosed by its lobular arrangement, presence of reserve cell hyperplasia and squamous metaplasia, low mitotic activities, and associated inflammation in the stroma.
Problem arises when the lesion becomes florid and contains more cytological atypia and even signet ring cells. The hyperplastic glands are arranged in reticular or solid patterns mimicking stromal invasion. Compounding the situation even further is when microglandular hyperplasia is involved by glandular dysplasia. In both situations, the presence of typical areas provides an important clue. Immunostainings for CEA, p16, and ki67 offer additional help in its distinction from adenocarcinoma. To differentiate from microglandular variant of endometrial carcinoma involving the cervix, ER, PR, and vimentin stains can be applied. The hyperplastic epithelial cells are positive for ER but negative for PR and vimentin [21].
Tunnel Clusters
Two frequently admixed types of tunnel clusters exist in the cervix. Type A tunnel cluster contains close-packed glands lined by columnar cells which might exhibit nuclear enlargement and pleomorphism. However, their superficial location and well circumscription differentiate them from invasive carcinoma.
Type B tunnel clusters are grossly mucin-containing cysts which are lined by an attenuated epithelium. They share the superficial location and demarcation features of type A tunnel clusters. Nevertheless, these two types probably should be separated further since type A has been shown to be related to lobular endocervical glandular hyperplasia, a likely precursor to cervical adenocarcinoma [22].
Lobular Endocervical Glandular Hyperplasia
Lobular endocervical glandular hyperplasia represents a metaplastic process which is a precursor lesion to cervical adenocarcinoma independent of human papilloma virus infection [23, 24]. It is characterized by a lobular proliferation of mucinous glands with pyloric gland phenotype. The glands are centered around larger glands (ducts) and are confined to the inner half of the endocervix. Typically, there is no significant cytological atypia. A diagnosis of atypical LEGH is made nuclear by the presence of atypia, mitotic figures, or apoptotic body or loss of cell polarity.
LEGH NOS has been used to describe endocervical lesion which shows neither a lobular nor laminated appearance. Most of them are incidental findings.
Diffuse Laminar Endocervical Gland Hyperplasia
Rather than being a mass lesion, diffuse Laminar endocervical gland hyperplasia presents as an incidental finding [25]. This lesion is characterized by a laminar proliferation of small- or medium-sized glands limited to the inner 1/3 of the cervical wall. When affected by inflammation, nuclear atypia can be present. The fact that the glandular proliferation is circumferential and appears to stop at the same level in a straight line at the base suggests that it might result from incomplete involution of pregnancy-related glandular hyperplasia.
Mesonephric Remnants and Hyperplasia
Mesonephric remnants are small tubules in clusters and contain thyroid-like material. They are commonly located deep in the lateral wall. A transmural lesion can result when the remnants become hyperplastic. The lobular type of hyperplasia sometimes has a central ductal structure. The diffuse type has evenly distributed pattern without stromal reaction, even though mild atypia and even mitotic figures can be seen. The tubular epithelium is positive for PAX2 and negative for CEA [26].
Endocervicosis, Endometriosis, and Endosalpingiosis
Endocervicosis in the cervix presents in the outer wall as variably shaped and sized mucinous glands which can become cystic. The extravasation of mucin can induce reactive changes. Thus, lesion is differentiated from adenocarcinoma by the presence of normal uninvolved stroma between the lesion of the surface and superficial normal glands.
Endosalpingiosis has the same anatomical location as endocervicosis, and the same criterion can be used in its distinction from invasive carcinoma.
Endometriosis of the cervix should not be confused with tubo-endometrioid metaplasia (discussed earlier). It is confined to the superficial one third of the cervical wall and contains both endometrial glands and stroma resembling proliferative endometrium.
The Uterus
Review of Pertinent Histology and Physiology
The uterus is an important organ in the reproductive system in that it is the actual site where a new life is formed and develops to term. In order to accommodate the inception, growth, and subsequent expulsion of the new life, the three major structural components of the uterus are brought under the coordinated influence of steroid hormones [27].
The Uterine Mucosa
The mucosa of the uterine body can be divided into three functional/histological layers. During reproductive years, the epithelial and stromal cells in the spongiosa undergo dramatic, cyclic changes in the repetitive business of preparing fresh receptive soil in anticipation of a possible implantation. In the process, the uterine epithelium and stroma form a structural and functional unit mediated through steroid hormone and paracrine factors. It is now known that endometrial epithelial and stromal cells probably share a common stem cell pool which gives rise to a mucosa composed of roughly equal amount of each component at the end of the proliferative phase [27–29]. The normal proliferative glands are straight and nonbranching.
The Uterine Stromal Cells
In the functional and structural units, the stromal cells not only provide structural support but also exert important dual effects on the epithelium. In providing structural scaffold for the cyclic endometrium, the stromal cells produce an elaborate network of reticulin fibers for each of them and develop a close relationship with small arterioles which might even derive from the same stem cells as the stromal cells.
On the one hand, the uterine stromal cells secrete growth factors via estrogen receptor pathways to facilitate the epithelial growth. On the other hand, in response to progesterone, they inhibit exuberant epithelial proliferation in response to progesterone [27]. Lack of or defect in this inhibitory effect leads to endometrial hyperplasia and carcinoma with resultant glandular crowding, branching, and even confluent growth.
Regional Mucosal Variance and Plasticity
The uterine endometrium is known for its regional variations and epithelial plasticity. The basalis and the compact (surface) show little change in morphology during the menstrual cycle (caveat; the surface get shed and the basalis remain throughout the menstruum). The lower uterine segment has a thinner and sluggish mucosa and often contains hybrid endocervical/endometrial components. The epithelial plasticity manifests in the form of metaplastic changes which are frequently present in endometrial specimens [30].
The Myometrium
The uterine myometrium is different from the smooth muscle tissues in other organs in two major aspects. First, uterine smooth muscle cells are responsive to steroid hormones. As a result, cyclic changes do occur even though they are not dramatic as those of the endometrium. During pregnancy, the myometrium undergoes extraordinary increase in mass through hyperplasia and hypertrophy. Concurrently, the uterine vasculature proliferates and remodels resultant from concerted effect of high levels of steroids and trophoblasts [31–33]. Secondly, the uterine smooth muscle fibers also have the capability to produce extracellular matrix material such as collage and elastin. Some smooth muscle cells at the endometrium/myometrium junction can have a hybrid stromal/smooth muscle phenotype.
Key Morphological Feature of Well-Differentiated Endometrial Adenocarcinoma
Confluent growth pattern or complex papillary and villous glands
Minimal intervening or altered stroma (fibrosis) (Figs. 6.7 and 6.8)
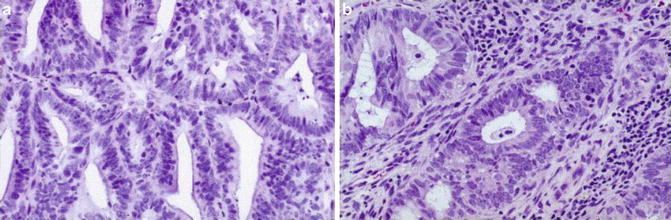
Fig. 6.7
Well-differentiated endometrial adenocarcinoma. Confluent growth pattern (a) and back-to-back glands (b) (Annals of Diagnostic Pathology, Elsevier/Churchill Livingstone, 2005 with permission)
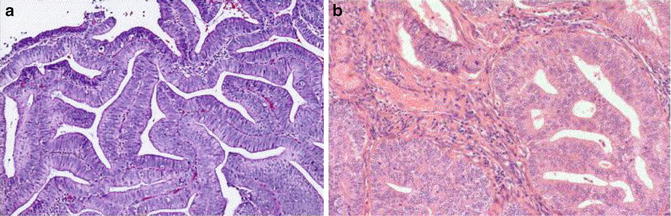
Fig. 6.8
Endometrial carcinoma. Villoglandular growth pattern (a) and altered stroma (b)
Discussion
Endometrial carcinoma develops from endometrial hyperplasia with PTEN mutation and PAX2 downregulation. The tipping point is reached when stromal invasion is evident. In this specialized organ where the epithelial and stromal components are derived from the same stem cells, stromal invasion manifests in three different forms [34]. They are (1) confluent glandular growth (or back-to-back glands) with no evident intervening stroma, (2) complex papillary or villoglandular structures, and (3) altered stroma.
Confluent glandular growth pattern manifests as complex branching or cribriform formations so that individual glands are surrounded by minimal or nil uterine stromal component. Complex papillary and villous glands are defined as branching papillary or villous structures with a fibrovascular core. Those intraglandular papillation and delicate papillae which are commonly present in metaplastic and hyperplastic processes should be excluded. To avoid overcalls, it is essential that the integrity of the specimen be not compromised.
In the evaluation of stromal alteration as evidence for stromal invasion, the shape and arrangement of the collagen-producing stromal cells are important features. They are more elongated, wavy, and eosinophilic than the normal stromal cells and are arranged in parallel fascicles compressed by collagen fibers. Caution should be exercised so that this stromal alteration is not confused with the fibrous stroma of endometrial polyps and tissue from the lower uterine segment. Endometrial polyps seem to be monoclonal stromal proliferation with secondary epithelial growth which is usually mild and seems undisturbed by stromal component. The stromal proliferation is frequently hyalinized and contains thick-walled blood vessels. The lower uterine segment stroma, particularly that of the inferior portion, is very fibrous. However, glandular proliferation in the form of glandular crowding and branching is lacking.
The grading of endometrial carcinoma employs a different set of criteria from those of most other adenocarcinomas. Well-differentiated carcinoma is defined as a tumor which contains less 5 % solid areas and lacks high-grade nuclei. In a case where the nuclei are markedly enlarged, hyperchromatic with prominent nucleoli, a higher grade is rendered (moderately differentiated, grade 2). Moderately differentiated carcinoma typically contains solid growth involving between 5 and 50 % of the tumor. Presence of marked nuclear atypia upgrades the tumor to grade 3 (poorly differentiated). Therefore, tumor necrosis and mitotic rate are left out of the grading system. In the evaluation of solid masses, it is very important that the squamous nests and sheets be excluded.
Similar to the nonneoplastic endometrial epithelium, the transformed epithelial cells can also manifest remarkable plasticity and the tumor shows frequent squamous, mucinous, ciliated, and even secretory and sex cord differentiation. Awareness of this important phenomenon avoids mistaking the differentiated components as benign tissue or confusing them as high carcinomas (such as clear cell carcinoma and serous cell carcinoma).
Finally, endometrial carcinoma, particularly in the well differentiated, can undergo dedifferentiation [35, 36]. Together with those entities showing sex cord differentiation (with spindle cells and hyalinized stroma) or containing benign chondroid and osteoid stroma, it needs to be differentiated from malignant mesodermal mixed tumor (MMMT). Malignant mesodermal mixed tumor (MMMT) contains separable high-grade epithelial and mesenchymal components.
Differential Diagnosis
Glandular Crowding Due to Stromal Breakdown or Artifact, Exaggerated Papillary Hyperplasia
Glandular crowding due to stromal breakdown or artificial displacement represents a common pitfall of overdiagnosis. In endometrial breakdown commonly seen in menstruation or anovulatory cycles, the epithelial structures lose the stromal support and become piled up with altered cellular polarity and even chromatin texture. Important clues to menstrual endometrium include secretory changes, predecidual changes in the stroma, nuclear dusts, and abundant neutrophils. In both menstruating and non-menstruating endometrium, endometrial tissue can be seen in the vessels. This should be interpreted as a sign of malignancy.
For small biopsies, crowding due to artifact can be recognized when the tissue adjacent to the displaced glands show signs of artificial shearing. Sometimes atrophic surface epithelium can aggregate and causes confusion with adenocarcinoma. It lacks true glandular crowding, epithelial stratification, or nuclear atypia.
Papillary hyperplasia is commonly seen on the surface of an endometrial polyp. Occasionally intracystic growth pattern is seen. The lining cells can undergo mucionous, eosinophilic, and ciliated metaplasia. The papillae can show branching and tufting. When present without a polyp, it can be misdiagnosed as endometrioid carcinoma or even papillary carcinoma. The nuclei are bland looking. No epithelial stratification nor real glandular crowding is evident.
Polyp, Mid- to Late Secretory Endometrium
Endometrial polyps can have irregular glands with focal glandular crowding. The crowding usually does not reach a level at which differentiation from carcinoma needs to be made. However, if involved by papillary hyperplasia, a differentiation is necessary.
Mid- to late secretory endometrium can have significant crowding. Since the cells show nuclear polymorphism and enlargement as well as loss of cell polarity when not accompanied by predecidual stroma, it can be mistaken as carcinoma in small biopsies when a predecidual stroma is evident.
Endometrial Hyperplasia
Compared to the proliferative endometrium, endometrial hyperplasia has an increased gland/stroma ratio with or without cytological atypia. With complex hyperplasia, the glandular structures can show significant branching and crowding. There is lack of the three criteria of endometrial carcinoma.
Atypical Polypoid Adenomyoma
The atypical polypoid adenomyoma typically presents in the lower uterine segment as a polypoid lesion. It contains irregular and haphazardly arranged endometrial glands in a fibromuscular stroma, simulating myometrium invasive endometrial carcinoma. The glands can show pseudostratification with mild to moderate cytological atypia. Abundant squamous morules are an important component, and they are frequently involved by necrosis. Important clues to this lesion are the polypoid presentation, short interlacing fibromuscular fascicles, and lack of endometrial stromal rimming.
Adenomyosis and Adenomyoma
Adenomyosis is defined as an endometrial tissue in myometrium. When a mass is formed, it is called adenomyoma. Typically the lesion contains both epithelial and stromal components even though either component can become inconspicuous. The important features of the lesion are its lobular appearance and hypertrophy of the surrounding muscle fibers. It lacks squamous morules as well as stromal desmoplasia.
Microglandular Hyperplasia and Endocervical Adenocarcinoma
Microglandular hyperplasia is a benign cervical lesion in young females. The morphology of the lesion can be mimicked by endometrial carcinoma with microcystic structures, focal squamous metaplasia, and inflammatory infiltrate. The malignant glands have, however, more glandular complexity and cytological atypia (Fig. 6.9).
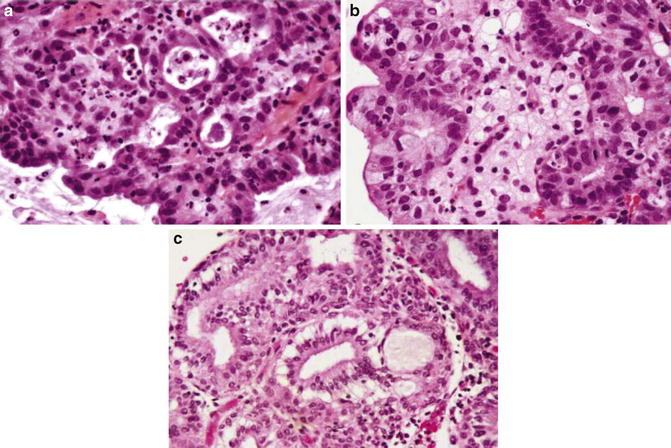

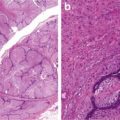
Fig. 6.9
Adenocarcinoma, microglandular variant (a, b). Note mitotic figures and foamy cells (b). Microglandular hyperplasia (c) (Diagnostic Gynecologic and Obstetric Pathology, Elsevier/Saunders, 2011 with permission)
Occasionally, a distinction of endometrial carcinoma from endocervical adenocarcinoma is needed in biopsy or curettings specimens. The distinction can be made easier by employing a panel of immunostainings (ER, PR, p16, and vimentin). Endometrial adenocarcinoma is diffusely positive for ER, PR, and vimentin and focally positive for p16. As the vast majority of endocervical adenocarcinomas are HPV related, they are usually negative for ER, PR, and vimentin. Instead, the tumor cells manifest diffuse reactivity to p16 antibody.
Key Morphological Feature of Low-Grade Uterine Stromal Sarcoma
Myometrial or vascular invasion (Fig. 6.10)
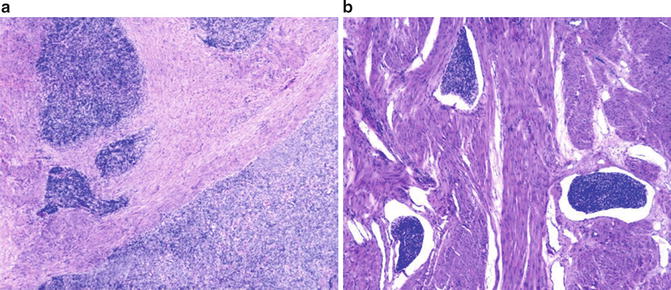
Fig. 6.10
Low-grade uterine stromal sarcoma. Myometrial (a) and vascular invasion (b) (Diagnostic Gynecologic and Obstetric Pathology, Elsevier/Saunders, 2011 with permission)
Discussion
Uterine stromal sarcomatous cells overexpress Bcl-2 probably as a result of chromosomal rearrangement, and they typically show low mitotic activity and negligible tumor cell necrosis and/or apoptosis. In contrast to the parameters used in the evaluation of uterine leiomyomatous lesions, muscle or vascular invasion becomes the yardstick in the differentiation between a benign stromal nodule and stromal sarcoma. Typically, myometrial invasion is in the form of tentacles, but may also present as an intramyometrial nodule or rarely diffuse myometrial thickening with no evident tumor mass formation. While uterine stromal cells develop a close functional/histological relationship with arterioles, sarcomatous cells tend to invade lymphatics and veins.
Uterine stromal lesions frequently show divergent differentiations, and unfamiliarity with this important feature could lead to erroneous diagnoses. For instance, frequent sex cord elements, glandular structures, and heterologous elements can be seen. Stromal hyalinization and smooth muscle differentiation can also be present resulting in a diagnosis of mixed stromal–smooth muscle tumor.
Adenosarcoma and adenofibroma can also show sex cord differentiation and heterologous elements. They can, however, be differentiated from low-grade endometrial stromal sarcoma with glandular differentiation by the presence of their leaflike growth pattern. Adenosarcoma differs from adenofibroma in that it has periglandular condensation of stromal cells which have increased cellularity, mitotic activity, and cytological atypia [37]. This is in stark contrast to mammary phyllodes tumors in which periglandular stromal cell condensation is not a sign of malignancy. Instead, malignant phyllodes tumor is characterized by marked stromal overgrowth. Only a small fraction of adenosarcomas share this feature.
Differential Diagnosis
Endometrial Stromal Nodule
An endometrial stromal nodule is composed of uniform, small cells with scant to moderate cytoplasm and poor cellular borders. Glandular and sex cord-like elements can be present. The lesion, however, lacks invasion into the endometrium, myometrium, or vessels. Importantly, an extension or protrusion of less than 3 mm into the adjacent tissue is not considered invasion. As this stromal lesion can frequently show smooth muscle and fibrous differentiation, it is also important that the intermingling metaplastic smooth muscle fibers and stromal cells are not interpreted as evidence of myoinvasion.
Cellular Leiomyoma and Intravenous Leiomyomatosis
Highly cellular leiomyoma can be confused with uterine stromal sarcoma in small biopsy/curettage specimens because the tumor border cannot be evaluated. Cellular leiomyoma is composed of larger spindle-shaped smooth muscle fibers. Important clues include large thick vessels in the lesion.
Intravenous leiomyomatosis (IVL) can mimic uterine stromal sarcoma in that it invades vessels and dissects into adjacent myometrium. The tumor cells, however, are larger and tend to show a predominant intravascular component. Immunostaining for SMA, desmin, caldesmon, and CD10 and even reticulin stain can help make the distinction.
Adenomyosis and Adenomyoma
Two variants of adenomyosis are particularly prone to be confused for stromal sarcoma. In one variant, the endometrial tissue has a propensity to protrude into the myometrial vessels. However, this is not a true vascular invasion. Immunostainings for endothelial cells would reveal a complete layer of endothelium covering the endometrial tissue.
The other variant has an inconspicuous glandular component, and its stromal component maintains a lobular appearance with hypertrophy of adjacent smooth muscle fibers. Typical adenomyosis areas can be found in other location.
Adenomyoma is a well-circumscribed mass composed of endometrial stroma, glands, and smooth muscle fibers.
Key Morphological Feature of Well-Differentiated Uterine Leiomyosarcoma
Tumor cell necrosis and mitotic figures >10/10HPF
Or tumor cell necrosis with significant nuclear atypia (Figs. 6.11 and 6.12)
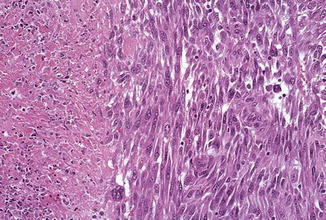
Fig. 6.11
Leiomyosarcoma with tumor necrosis (Journal of Clinical Pathology, American Society of Clinical Pathology, 2007; Best Practice & Research in Clinical Obstetrics and Gynecology, Elsevier/Churchill Livingstone, 2011 with permission)
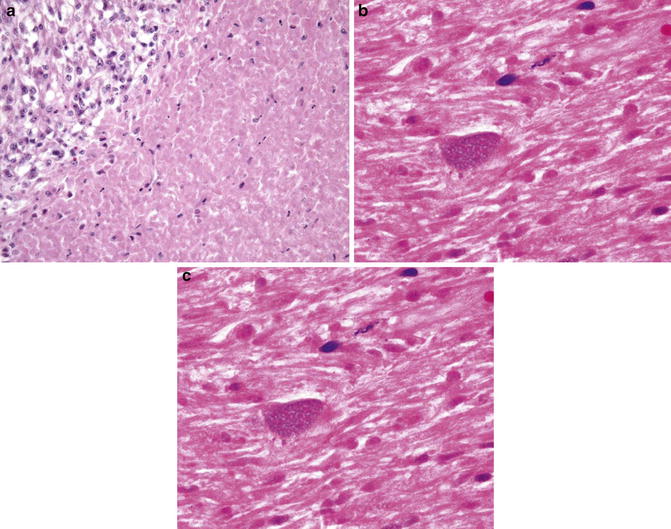
Fig. 6.12
Tumor necrosis. Note abrupt and sharp interface between necrotic and viable tissue. Ghost cells present (Diagnostic Gynecologic and Obstetric Pathology, Elsevier/Saunders, 2011 with permission)
Discussion
The uterine smooth muscle tissue (myometrium) is programmed to respond to steroid hormones in order to provide a muscular conglomerate for labor. During pregnancy, the uterine smooth muscle tissue undergoes tremendous increase in mass through cellular hyperplasia and hypertrophy. Understandably, there should be multiple protective mechanisms to keep this extraordinary growth potential in check in nongravid conditions. Commonly encountered uterine leiomyomas are characterized by a variety of simple genetic changes involving multiple pathways (mechanisms) [38]. The multiple involved pathways might account for the many variants of uterine leiomyoma. Some of them show concerning features and they include mitotically active, cellular, atypical (with bizarre nuclei), dissecting, vascular invasion and even metastasizing variants.
The predominant view holds that most leiomyosarcomas arise de novo and they contain far more complex cytogenetic changes than leiomyomas. These complex chromosomal abnormalities lead to genomic instability and knockdown of the protective mechanisms, allowing aggressive growth, recurrence, and frequent metastasis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree