The relative contributions to CRF of treatment with radiation, chemotherapy, hematopoietic stem cell transplantation, and hormonal, biologic, and molecularly targeted agents have been explored.6,8,10,39,48–52 Fatigue symptoms are known to fluctuate across the cancer treatment course,53 although few relationships between treatment-related variables such as dose-intensity, schedule, and time since treatment completion have been observed.2 Comparatively less is known about the association between surgical treatments and CRF.54 During a course of fractionated radiation therapy, fatigue is often cumulative and peak severity may occur after radiation is concluded.55
Although the precise pathophysiology is poorly understood, studies suggest that fatigue may be related to anemia; mood disorder; concurrent symptoms such as pain, sleep disturbances, electrolyte disturbances, cardiopulmonary, hepatic or renal dysfunction, hypothyroidism, hypogonadism, adrenal insufficiency, infection, dehydration, protein-calorie malnutrition, cachexia, asthenia, and deconditioning; and the side effects of drugs such as opioid analgesics or anticonvulsants that act on a central nervous system.43,50,52,56–65 Altered energy metabolism within skeletal muscle has been postulated to cause CRF. Both the tumor itself and treatment with radiation or chemotherapy can produce anemia or cachexia, and both of these may contribute to altered skeletal muscle energy metabolism, disrupted mitochondrial synthesis of ATP, or diminished oxygen delivery to muscle cells.66 Treatment with radiation or chemotherapy can produce anemia or cachexia, and both of these may contribute directly or indirectly to altered skeletal muscle energy. Accumulating evidence also suggests that gene polymorphisms, disruptions in the hypothalamic-pituitary-adrenal (HPA) axis, altered circadian rhythmicity, immune dysregulation, altered proteomic pathways, and proinflammatory cytokine activity may directly or indirectly contribute to CRF.46,67–73 Associations between the occurrence and severity of CRF and demographic variables such as gender, age, marital status, and employment status have not been consistently identified.2 In any one individual, the etiology of CRF is likely to be multifactorial, and contributors across the disease trajectory may fluctuate.
CURRENT THEORIES OF CANCER-RELATED FATIGUE
Several different explanatory models of CRF have been proposed. Many of these models use similar constructs. Conceptual models can be organized into four thematic groups: (1) energy balance/energy analysis models, (2) fatigue as a stress response models, (3) neuroendocrine-based regulatory fatigue models, and (4) hybrid models.
Energy balance/energy analysis models depict energy as the major variable in fatigue and alterations in the balance among intake, metabolism, and expenditure of energy as factors in producing fatigue. Examples of this thematic group of models include the integrated fatigue model of Piper et al.,74 the energy analysis model of Irvine et al.,75 and the psychobiologic-entropy model of Winningham.76 Fatigue as a stress response models posit that tiredness, fatigue, and exhaustion form an adaptational continuum of response to stress. Each state along this continuum from tiredness to exhaustion may be distinguished by different behavioral and symptom patterns. Examples of models included in this thematic class include fatigue models proposed by Aistars,77 Rhoten,78 Glaus,79 and Olson.80 Neuroendocrine-based regulatory fatigue models hypothesize that the multiple dimensions of fatigue are explained by dysregulation in the function of neuroendocrine-based regulatory systems including the hypothalamic–pituitary axis, circadian rhythms, and neuroimmune system transmitter secretion and function.81 Examples of models based on neuroendocrine dysregulation include those that have been proposed by Lee et al.,82 Payne,83 and Schubert et al.71 Models that represent hybrid conceptual approaches have also been proposed. Olson et al.84 have recently proposed a model of CRF suggesting that stressors associated with cancer and its treatment trigger declines in four systems—cognitive function, sleep quality, nutrition, and muscle endurance—and that these declines reduce one’s ability to adapt. Al Majid and Gray85 have also proposed a hybrid model that incorporates biologic, psychobehavioral, and functional variables implicated in the induction of fatigue, and have illustrated the application of this model to define the mechanisms by which exercise may ameliorate CRF.
Models in all four thematic classes may be helpful in generating testable hypotheses for continued research into the problem of CRF and in guiding the development and evaluation of interventions to limit and manage fatigue, and to reduce its deleterious impact on health-related quality of life.
EVALUATION OF THE PATIENT WITH CANCER-RELATED FATIGUE
Studies suggest that CRF is underdiagnosed, that the assessment of fatigue in patients with cancer is suboptimal, and that health-care professionals may not fully appreciate the degree of distress and functional loss that fatigue produces.86–90 Identified barriers to communication between patients and their clinicians about fatigue include a tendency to view fatigue as an inevitable consequence of illness, clinicians’ failure to offer interventions, patients’ lack of awareness of effective treatments for fatigue, a desire on the patient’s part to treat fatigue without medications, and a tendency to be stoic about fatigue to avoid being labeled as a complainer and to avoid prompting a change in therapy toward less active/aggressive treatments.86,91–93 Instruments to evaluate patient-derived barriers to fatigue management,94,95 self-efficacy for fatigue management,96 and the contributing factors to fatigue58 have been proposed.
Identifying patients with CRF is the first key step in improving fatigue evaluation and management. Although there is currently no consensus concerning the optimal method or frequency to screen for CRF in the clinical or research setting,97–99 evidence of the widespread occurrence of CRF suggests that routine screening for CRF at regular intervals throughout treatment, follow-up, and long-term follow-up is warranted. Technologies that use electronic or Web-based assessment routines, including computer-adapted testing (CAT), can streamline screening procedures and integrate fatigue screening into workflow.100,101
There is accumulating evidence that single-item measures to screen for fatigue are rapid and sensitive, and can be applied efficiently in the clinic to identify patients who would benefit from more systematic evaluation.102 Based on National Comprehensive Cancer Network (NCCN) guidelines,103 fatigue should be assessed quantitatively on a 0 to 10 scale (0 = no fatigue and 10 = worst fatigue imaginable); those patients with a severity of more than 4 should be further evaluated by history and physical examination.
Although a single-item measure may provide for a rapid assessment of general fatigue or serve as a screening tool, evidence suggests that single-item measures do not fully capture all the dimensions of fatigue.104,105 There is good consensus in the literature that fatigue generally consists of a sensory dimension (fatigue severity, persistence), a physiologic dimension (e.g., leg weakness, diminished mental concentration), and a performance dimension (reduction in performance of needed or valued activities). More than 20 self-report measures (including single-item measures, multi-item unidimensional scales, and multidimensional inventories) have been developed to measure fatigue in patients with cancer.106–110 Consideration of the measurement properties and strengths and limitations of these instruments, including reliability, validity, specificity, sensitivity to change, recall period, respondent burden, translation in multiple languages, and the availability of normed values to aid interpretation, should be used to guide decisions about the utility of a measure for specific clinical or research purposes.97,111–113 Ecologic momentary assessment (a technique that offers real-time measurement of a phenomenon as it occurs in a naturalistic setting) may overcome some of the methodologic limitations of fatigue assessment, including recall bias and the influence of current context on the self-report of fatigue.114
A detailed history in patients with moderate or severe CRF includes the presence, intensity, and pervasiveness of fatigue, its course over time, the factors that exacerbate or relieve fatigue, and the impact of fatigue on functioning and level of distress. Clinicians can obtain valuable information about the consequences of CRF by exploring the effects of CRF on self-esteem, mood, and the ability to perform activities of daily living; fulfill important roles as parent, spouse, and worker; and relate to family and friends. Also important to the evaluation is an assessment of what interventions the patient is using to manage fatigue and the effectiveness of them. The dimensions of the fatigue experience that should be explored when evaluating a patient with CRF are listed in Table 142.2.

In evaluating the patient with CRF, it is important to screen for treatable etiologic or potentiating factors including hypothyroidism, hypogonadism, adrenal insufficiency, cardiomyopathy, pulmonary dysfunction, anemia, sleep disturbance, fluid and electrolyte imbalances, emotional distress, physical inactivity, deconditioning, and uncontrolled concurrent symptoms.10,57,115–118 The medication profile should also be reviewed to identify specific classes of medications such as opiates, antidepressants, anticonvulsants, antiemetics, antihistamines, and beta blockers, and drug–drug interactions that can also intensify fatigue.19,103,119,120
Because fatigue typically has several different causes in any one patient, the treatment plan needs to be individualized. It is helpful to work with the patient and family caregivers to improve the assessment of fatigue and identify management strategies. Open communication between the patient, family, and caregiving team will facilitate discussion about the experience of fatigue and its effects on daily life. General supportive care recommendations for patients with fatigue include encouraging a balanced diet with adequate intake of fluid, calories, protein, carbohydrates, fat, vitamins, ABD minerals and balancing rest with physical activity and attention-restoring activities such as exposure to natural environments and pleasant distractions such as music.103
There have been more than 210 empiric studies of pharmacologic and nonpharmacologic interventions to reduce or manage CRF and a number of meta-analyses or systematic reviews.121–134 These are summarized in Table 142.3, and selected findings are discussed here. Guidelines for the management of cancer-related fatigue have been disseminated by NCCN103 and the Oncology Nursing Society.135
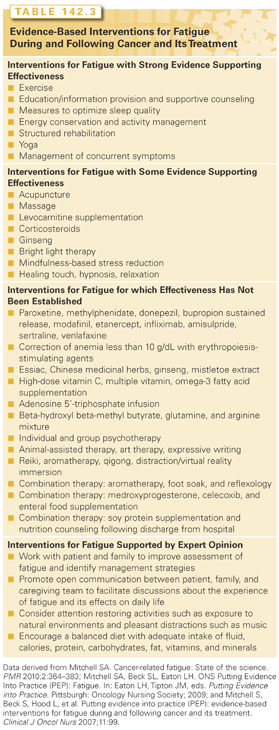
Several pharmacologic agents (including paroxetine, venlafaxine, methylphenidate, donepezil, bupropion, and modafinil) have been evaluated for their effectiveness at reducing fatigue during and following cancer treatment.122,126,136 Four trials have examined the effectiveness of paroxetine in treating fatigue during and following cancer treatment with mixed results. In three multicenter, randomized, double-blind, placebo-controlled trials, paroxetine 20 mg by mouth daily did not demonstrate a beneficial effect on fatigue outcomes, although improvements in depression and overall mood were noted in the paroxetine treatment group.137–139 However, two small trials show a trend toward a possible benefit for either paroxetine140 or venlafaxine141 in treating fatigue in women experiencing hot flashes.
Two recent meta-analyses and a systematic review concluded that there is preliminary evidence supporting the use of methylphenidate to treat CRF, although more study of efficacy and tolerability was warranted.127,142,143 Recent evidence from a meta-analysis across trials suggests that the D-isomer form of methylphenidate may be more effective than other forms, and that patients with more severe and those demonstrating an improvement in fatigue within the first few days of methylphenidate treatment are the most likely to derive benefit.144
Two small trials also suggest that donepezil 5 to 10 mg per day145,146 or bupropion sustained release at a dose of 100 to 150 mg per day147,148 may be effective at limiting fatigue. However, in a controlled trial of donepezil, improvements in fatigue outcomes were not observed.149 Several trials also suggest that modafinil at a dose of 100 mg twice a day may be effective at treating fatigue and improving daytime wakefulness and cognitive function in patients during and following cancer treatment.150–153 Additional rigorously designed trials of donepezil, bupropion, and modafinil for fatigue appear warranted.
Several nutritional supplements including coenzyme Q10, levocarnitine, lectin standardized mistletoe, omega-3 fatty acid supplements, ginseng, guarana, and valerian have been explored, either as single agents or as part of a combination therapy.130,154–156 Several trials suggest that levocarnitine supplementation is effective in patients who have low serum carnitine levels.157–161 However a large double-blind, placebo-controlled trial failed to demonstrate an improvement in fatigue outcomes in patients with malignancy and good performance status, even in those study participants who were levocarnitine deficient.162 Guarana 50 mg twice daily has been shown to be safe and effective at treating CRF in women with breast cancer in a randomized placebo-controlled cross-over study (n = 75)163; however, guarana 75 mg once daily was not effective at treating fatigue in women with breast cancer who were undergoing radiation therapy.164 The primary active ingredient in guarana is caffeine. A week course of Wisconsin ginseng dosed at 2,000 mg daily was effective at improving fatigue outcomes in cancer survivors and was well-tolerated in a large (n = 364) double-blind, placebo-controlled trial.165
Low-dose dexamethasone at a dose of 4 mg twice daily for 2 weeks improved fatigue outcomes in a randomized placebo-controlled trial in 84 patients with advanced cancers.166 Although adverse events were comparable in the dexamethasone and placebo groups, systemic corticosteroids could be associated with a prominent adverse effects profile in particular subpopulations such as those at the end of life.167 A pilot, randomized placebo-controlled cross-over study has also shown evidence for the effectiveness and tolerability of thyrotropin-releasing hormone in improving fatigue outcomes in a small sample of cancer patients with significant fatigue.168 Although male hypogonadism is hypothesized to adversely affect mood and well-being,169 a recent blinded, placebo-controlled trial of testosterone replacement therapy in a small sample of men with advanced cancer did not demonstrate positive effectiveness on fatigue end points.170
TREATMENT OF ANEMIA WITH ERYTHROPOIESIS-STIMULATING AGENTS
Although data from seven systematic reviews suggest that patients receiving erythropoiesis-stimulating agents (ESA) to correct severe anemia (hemoglobin less than 10 g/dL) may experience diminished fatigue and increased vigor,171 there is only limited evidence that ESAs improve fatigue when anemia is less severe.172–176 In addition, a recent systematic review concluded that in many of the trials, the improvement in fatigue outcomes did not exceed the minimally important clinical difference.177 Importantly, the use of ESAs for fatigue must be considered in light of safety issues, including an increased risk of thrombotic events, hypertension, and pure red cell aplasia, and concerns that ESAs may decrease local–regional disease control and survival outcomes in particular tumor types.173,175,178
National clinical practice guidelines179–181 and product labeling from the U.S. Food and Drug Administration182 should direct individualized management of patients with cancer- or treatment-associated anemia, including an analysis of the risks and benefits of ESAs versus packed red blood cell transfusions based on cancer treatment goals, and decisions about patient monitoring, treatment thresholds, dose reductions, treatment discontinuation, and the use of supplemental iron in patients receiving ESAs. Particular caution is needed when using ESAs in patients who are not receiving chemotherapy because recent trials report increased thromboembolic risks and decreased survival in this subpopulation.176,183–185
Meta-analyses of randomized trials support the benefits of exercise in the management of fatigue during and following cancer treatment in patients with breast cancer or solid tumors, or who are undergoing hematopoietic stem cell transplantation, although effect sizes are generally small, and positive results for the outcome of fatigue have not been observed consistently across studies.132,186–189 The exercise modalities that have been applied differ in content (walking, cycling, swimming, resistive exercise, or combined exercise), frequency (ranging from two times per week to two times daily), intensity (with most programs at 50% to 90% of the estimated VO2 maximum heart rate), degree of supervision (fully supervised group versus self-directed exercise), and duration (from 2 weeks up to 1 year). Knowledge about the type, intensity, and duration of physical exercise that is most beneficial for reducing fatigue at different stages of disease and treatment is not known,190 and more research is needed to systematically assess the safety of exercise (both aerobic exercise and strength training) in cancer subpopulations.191
MANAGEMENT OF CONCURRENT SYMPTOMS
A recent large randomized trial demonstrated that effective management of concurrent symptoms including pain, shortness of breath, insomnia, and depression also produces improvements in CRF outcomes.38,118 Four other RCTs (n = 200 cancer patients with major depressive disorder192; n = 83 cancer survivors with fatigue193; n = 45 women with metastatic breast cancer who were depressed194; n = 78 advanced cancer patients with fatigue118 and a small case series (n = 6 women with metastatic breast cancer)195 as well as a systematic review,128 confirm that cognitive-behavioral therapy (CBT) interventions for symptoms may also alleviate CRF, including fatigue that occurs as a component of a symptom cluster along with pain and insomnia.
PSYCHOEDUCATIONAL INTERVENTIONS
Several adequately powered randomized controlled trials suggest that educational interventions; cognitive-behavioral treatment for fatigue, depression, and other symptoms; and psychological support have a role in supporting positive coping in patients with fatigue.121,124,196,197 Psychoeducational interventions that have been shown to be effective include anticipatory guidance about patterns of fatigue and recommendations for self-management, counseling, and supportive psychotherapy, and coordination of care. Energy conservation and activity management is a self-management intervention that teaches patients to apply the principles of energy conservation and activity management and provides coaching to integrate these activities into their daily lifestyle.
INTERVENTIONS TO IMPROVE SLEEP QUALITY
Studies indicate that cognitive-behavioral interventions designed to improve sleep quality also have a beneficial effect on fatigue.198–201 In contrast, the efficacy of pharmacologic therapies in treating fatigue by reducing insomnia has had very little systematic study.202 Cognitive-behavioral interventions to improve sleep quality can be delivered individually or in a group setting, and include relaxation training along with sleep-consolidation strategies (e.g., avoiding long or late afternoon naps, limiting time in bed to actual sleep time), stimulus control therapy (e.g., go to bed only when sleepy, use bed/bedroom for sleep and sexual activities only, set a consistent time to lie down and get up, avoid caffeine and stimulating activity in the evening), and strategies to reduce cognitive-emotional arousal (e.g., keep at least an hour to relax before going to bed, establish a presleep routine to be used every night). Morning exposure to bright light has been shown in two small randomized trials to prevent deterioration in CRF during chemotherapy for breast cancer,203,204 potentially by protecting recipients from circadian rhythm desynchronization during cancer treatment.205
Several trials206–211 and two systematic reviews212–214 support a conclusion that structured rehabilitation programs delivered formally by a multidisciplinary group result in statistically significant and sustained improvements in fatigue, particularly in patients who have completed treatment and are in the survivorship phase. However, because at least one study215 suggests that programs that are too intensive may actually worsen fatigue, tailoring based on the patient’s current level of energy and stage along the treatment trajectory is important.
There is preliminary evidence to support the efficacy of integrative approaches to the treatment of fatigue, including yoga, relaxation, mindfulness-based stress reduction, acupuncture, medical qigong, massage, healing touch, and Reiki, and combined-modality interventions that include aromatherapy, lavender foot soaks, and reflexology.125,128 The design of these studies tend to be open label and/or uncontrolled, with no random assignment, and with sample sizes that were extremely small, making it difficult to draw firm conclusions about efficacy. Despite these limitations, and with acknowledgment that the inclusion of controls such as double-blinding presents methodologic challenges,216 results suggest that these complementary therapies have potential in the treatment of fatigue in patients with cancer.
Yoga practices have shown particular effectiveness at improving fatigue outcomes in two rigorously conducted RCTs in breast cancer survivors.217,218 Yoga may also have beneficial effects in reducing fatigue in other populations.219–224 However, four systematic reviews223–226 have concluded that the effectiveness of yoga on fatigue outcomes has not been consistently established across a wide range of patient populations and at all points in the cancer trajectory. In addition, the high risk of bias across studies with respect to sample selection and blinding of participants and outcome assessors was also noted.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








