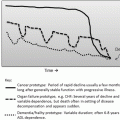
Organ system
Age-associated physiologic changes
Implications
Cardiovascular
Decrease in maximal heart and ventricular compliance and increase in vascular stiffness
Increase risk of heart failure during stress and increase risk of drug-induced cardiomyopathy
Gastrointestinal
Alteration in mucosal protective mechanisms.
Reduced colonic motility
Decline in hepatic drug metabolism
Susceptibility to mucositis leading to compromised nutrition
Increase risk of constipation
Variable absorption of drugs
Susceptibility to adverse drug reactions
Pulmonary
Increase in lung compliance
Increase in stiffness of chest wall
Diminished cough reflex
Diminished function of the mucociliary escalator
Decrease in pulmonary reserve
Increase risk of aspiration
Increased susceptibility to pulmonary infections
Renal
Decrease in glomerular filtration rate
Decrease in renal blood flow; reduced response to ADH; sodium wasting
Decrease in tubular function and hyporeninemic hypoaldosteronism
Nephrotoxicity from renally excreted drugs
Increase risk of volume depletion
Increase risk of electrolyte disturbances
Nervous/Cerebrovascular
Decrease in number of neurons
Impairment in vision, hearing and olfaction
Increase incidence of peripheral neuropathy
Impairment in response to postural change in arterial pressure and cerebral blood flow
Increase risk of impairment in memory and cognition
Increase risk of anorexia due to decrease in olfaction
Increase risk of delirium due to impairment in cognition, hearing, and vision
Increase risk of developing peripheral neuropathy or worsening of existing neuropathy
Increase susceptibility falls due to orthostatic hypotension and neuropathy
Hematologic
Decrease in bone marrow reserve
Increase risk of developing anemia, thrombocytopenia, and febrile neutropenia
Endocrine
Increase in osteoclast over osteoblast function
Altered temperature regulation
Increase risk of falls and fractures
Decrease in febrile response to infection
Musculoskeletal
Loss of muscle mass and strength
Loss of mobility
Impairment in gait and balance increasing fall risk
26.2.2 Multimorbidity and Polypharmacy
The likelihood of multiple chronic health conditions, referred to as multimorbidity, increases with age [7]. Comorbidity burden affects life expectancy, risk of functional decline, and hospitalization risk [8–11]. Increasing multimorbidity impacts survival and treatment tolerance in older adults with cancer [12–14]. The Charlson Comorbidity Index (CCI) is widely used in geriatric oncology research to characterize comorbidity burden. The CCI weights 19 diseases from one to six points based on relative risk of death at 1 year [15]. Higher overall mortality is associated with CCI score of 3 or more in patients with lung, colorectal, and prostate cancer who are 70 years and older [16].
There are also potential interactions between existing chronic diseases, a new diagnosis of cancer, and treatment. For example, the risk of falls with chemotherapy such as taxanes is higher in patients with pre-existing diabetes or peripheral neuropathy [17]. With a diagnosis of cancer, older patients are at higher risk for drug–drug interactions as the number of medication increases to treat the disease and manage symptoms [18]. Potential complications and side effects of treatment should be anticipated to make appropriate adjustment to current medications. For example, blood pressure medications, especially diuretics, may need to be reduced or held during periods of poor nutrition and dehydration due to nausea and vomiting. A careful review of medications for all patients at the beginning of treatment and periodic medication reconciliation is a practical approach to polypharmacy in the oncology setting [18]. The Beers Criteria lists potentially inappropriate medications for older adults . Other screening tools that are increasingly used in geriatric oncology to appraise medications for older patients are the STOPP (Screening Tool of Older Persons’ Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment) . (Chapter 5 provides details on the Beers Criteria and STOPP/START).
26.2.3 Functional Impairment/Malnutrition/Falls : Implications for Cancer Care
Functional impairment (limitations in ADL and IADL), frailty, and geriatric syndromes are common in older adults with cancer [19]. Impairments in IADL predict survival in older patients with cancer [20]. Patients with impairment in IADLs should be further assessed for impairments in cognition, physical performance, and activities of daily living (ADL) . Weight loss and malnutrition are associated with chemotherapy toxicity and decreased survival [21–23]. Treatment side effects such as nausea, vomiting, and mucositis can lead to dehydration and further weight loss. Fatigue can impair the ability to shop, prepare, and enjoy food. One third of patients 65 years and older fall at least once a year and up to half of those who fall have recurrent falls [24]. Treatment side effects such as neuropathy and advanced cancer stage increases the risk of falls in older patients with cancer [25]. Patients should be asked about falls or near falls in the last 6 months. (Further assessment for falls is described in the Assessment Chap. 8).
26.2.4 Geriatric Syndromes and Their Interplay with Cancer
Geriatric syndromes are common health conditions in older adults. The etiology is characteristically multifactorial, with shared risk factors including older age, comorbidity burden, cognitive decline, functional impairment, and impaired mobility [26]. In geriatric oncology, the most relevant syndromes are frailty, falls, dementia, depression, and delirium [27]. (Chapter 1 provides a full description of frailty and validated assessment tools.)
Cancer treatment decisions are complex, especially for older patients. The treatment program typically involves multiple office visits and complex medication regimens. The assessment of cognition informs the provider of a patient’s decisional capacity, reliability of history, ability to understand and manage complex treatment plans and the insight to report toxicities [28]. Patients with cognitive impairment need close monitoring for toxicities, such as febrile neutropenia. The prevalence of depression in older cancer patients ranges from 17 to 25 % [29]. Depression is under-recognized and under-treated, in part due to the overlap of symptoms of cancer and cancer treatment (fatigue and anorexia) and the signs and symptoms of depression. Delirium is also common in patients with cancer, with risk factors including polypharmacy, fevers, anemia, fatigue, pain, and electrolyte disturbances. (Chapter 2 provides a full description of this syndrome and the Chap. 8 Tools for Assessment provides details on assessment using the Confusion Assessment Method.)
26.2.5 Geriatric Assessment: Evaluating the Older Patient with Cancer
There is heterogeneity in physiological reserve, comorbidities, functional abilities, and presence of geriatric syndromes among older individuals, adding complexity to estimation of life expectancy and treatment management decisions. Geriatric assessment (GA) is a multidimensional assessment of an older patient’s health, fitness, and capabilities using validated tools. Potential components of GA include the following health domains: (1) medical: evaluation of comorbidity, polypharmacy, and nutritional status; (2) mental health: evaluation of cognition, depression, and delirium; (3) functional status: assessment of activities of daily living (ADL), instrumental activities of daily living (IADL), mobility (physical performance), and falls; (4) social: evaluation of environment, resources, and social support/network. There is a growing body of evidence on the utility of GA in oncology practice [30]. Many studies in geriatric oncology propose the use of GA in patients older than 70 years with cancer [30]. The ultimate goal of GA is to guide treatment management decisions and the design of a treatment plan that balances benefits and remaining life expectancy, anticipates complications and care needs, and implements targeted interventions to optimize outcomes and improve quality of life.
GA can be applied to help with clinical decision-making in various clinical scenarios including: (1) prior to cancer surgery to assess for risks and potential post-operative complications such as functional impairment, (2) to estimate life expectancy in the context of competing comorbidities and functional status, particularly in the setting of adjuvant chemotherapy, (3) to evaluate the risks and benefits of treatment options, (4) and to monitor for development of deficits as a result of cancer treatment during and post treatment [31]. Chapter 8, Tools for Assessment, describes validated tools to assess geriatric domains. Table 26.2 summarizes the assessment tools of value in older patients with cancer.
Table 26.2
Summary of geriatric assessment tools important in oncology
Assessment domain | Tools |
|---|---|
Comorbidity | Charlson comorbidity index (CCI) |
Polypharmacy | Medication reconciliation prior to treatment and periodic review Review of high risk medications based on BEERS Criteria and STOPP/START |
Nutrition | Mini-Nutritional Assessment (MNA) |
Cognition | Mini Cog Mini-mental state examination (MMSE) Montreal cognitive assessment (MoCA) |
Depression | Patient health questionnaire-2 (PHQ-2) |
Delirium | Confusion assessment method (CAM) |
Function | Katz index of activities of daily living (Katz ADL Index) Lawton instrumental activities of daily living (Lawton IADL Index) |
Mobility/Falls | Timed up and go test (TUG) Gait speed |
Social | Assess socioeconomic status, family care system, environment and advanced care planning |
26.2.6 Geriatric Assessment: Impact on Cancer Care
26.2.6.1 Detection of Important Geriatric Problems
Traditional oncology assessments miss important problems in older patients with cancer. For example, over half of older patients with an Eastern Cooperative Oncology Group Performance Score (ECOG PS) who are classified as “fit” (scores of 0–1) still have impairments of instrumental activities of daily living (IADL) [32]. GA detects impairments in greater than 50 % of older patients with cancer (n = 1967, Median age 76 years); the most frequent problems are impairment in function, nutrition, and fatigue [33].
26.2.6.2 Prediction of Chemotherapy Toxicity
There are two chemotherapy toxicity risk models for older adults with cancer. The Cancer and Aging Research Group (CARG) (based on 500 subjects with mean age 73 years) model found 11 factors that were predictive of Grade 3–5 chemotherapy toxicity [34]. GA assessment variables in this model were: hearing impairment, history of falls, needing assistance with medication management, limited ability to walk one block, and a decrease in social activities due to health status. CARG model allows risk stratification dividing patients into low (0–5 points), intermediate (6–9 points), or high risk (10–19 points) of chemotherapy toxicity [34]. Similarly, the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) model predicts severe hematologic (Grade 4) and non-hematologic toxicity (Grade 3/4) in older cancer patients. In this model, IADL dependence predicts hematologic toxicity while self-rated health status, Mini-Mental State Exam score, and Mini-Nutritional Assessment score predicts non-hematologic toxicity [23].
26.2.6.3 Prediction of Survival
There are currently no life-expectancy prognostic models in geriatric oncology, although there are several such models based on GA variables available for general geriatric patients (Available on Eprognosis.com). These models estimate remaining life expectancy in the context of competing comorbidities and geriatric specific factors. Studies have demonstrated prognostic value of GA domains in specific oncology settings. For example, poor nutritional status on Mini-Nutritional Assessment and abnormal Timed Up and Go scores predict early death in older patients with various cancer types (n = 384) [35]. Similarly, poor nutritional status, impaired function, and comorbidity also predict interruption of chemotherapy and mortality in patients with solid malignancies receiving chemotherapy [36]. All-cause and breast cancer-specific death rate at 5 and 10 years are doubled in women with greater than three GA deficits (n = 660, stage I to IIIa breast cancer) [37]. Measures of physical performance predicted overall survival and 2-year progression to disability or death in older patients with cancer [38].
26.2.6.4 Estimating the Impact of Treatment on Older Adults
Side effects from treatment may potentiate geriatric problems. For example, anemia and fatigue, which are common in older patients without cancer [39, 40], are more likely to occur during cancer treatment [41]. Fatigue often impairs the ability to complete tasks of daily living (cooking, preparing food, shopping, and taking medications) and increase the risk for cognitive impairment and functional dependence [40, 42, 43]. Continued assessment of physical and cognitive function during and following treatment is important to continue to optimize outcomes.
26.2.7 Geriatric Assessment -Guided Interventions
To be effective, GA must be followed by appropriate interventions to address deficits. Unfortunately, data on the impact of GA-driven interventions in older patients with cancer is limited. However, studies in community-dwelling older patients without cancer have demonstrated effectiveness in improving outcomes [44]. Table 26.3 outlines potential interventions to address deficits identified during GA.
Table 26.3
Geriatric assessment-guided interventions
Geriatric assessment identified problems | Interventions |
|---|---|
Functional impairment | Assess social support and implement visiting nurse and home health services Evaluate cognition Referral to physical and occupational therapy Medication review, address vision impairment, Vitamin D status, and home safety evaluation |
Nutrition risk | Referral to dietician for nutritional assessment and recommendations Assess for depression, access to food and social isolation Consider home delivered meals |
Cognitive impairment | Review medications—minimize medications with higher risk of delirium Assess and treat depression and anxiety Assess ADL and IADL, medications, and driver safety Evaluate for cause of impairment including Vitamin B12, thyroid function, and brain imaging Identify healthcare proxy Delirium risk counseling Social work involvement for caregiver education |
Depression | Treatment with medication Consider counseling Suicide risk assessment |
Social support | Elicit support from caregivers or implement services such as transportation assistance, home health care, and home delivered meals Monitor caregiver stress |
Comorbidity/Polypharmacy | Pharmacy review of medications Consider drug–drug and drug–disease interactions Diabetes—avoid neurotoxic agents Heart failure—closely monitor volume status Kidney disease—avoid nephrotoxic agents |
26.2.8 Using Screening Tools to Target Patients for GA
Three screening tools have been proposed to identify patients most likely to benefit from GA. The data supporting the use of screening tools have primarily focused on predicting deficits during Comprehensive GA (CGA) which is considered the “gold standard” for detecting problems in vulnerable older people. The Vulnerable Elders Survey-13 (VES-13) is a 13-item survey including age, self-rated health, and functional status, and is scored from 0 to 13, with 13 being the worst. A score of greater than 3 identified vulnerable older adults at risk for mortality, morbidity, and hospitalization. Higher VES-13 scores predict death and functional decline in vulnerable community-dwelling older adults [45, 46]. VES-13 demonstrates high predictive value for having greater than two deficits on CGA in older patients with prostate cancer [47]. Another tool, the Geriatric-8 (G8) screening tool, includes age, self-rated health, nutrition, cognition, mobility, and polypharmacy, and is scored from 0 to 17, with 17 indicating better function. A score of 14 or less predicts at least one deficit on CGA domains in adults 70 years and older [48]. Finally, the National Cancer Network Guideline recommends using the Fried Frailty score to identify older patients in need of further assessment. See Chap. 1, for further discussion of this syndrome in older patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree