Ethics, Professional Values, and Legal Considerations in Radiation Oncology
The medical profession experienced a rise in socioeconomic stature in the United States during the 20th century.1 Physicians began to receive higher wages for their services and enjoy greater personal respect as providers of health. Although progress in medical science has improved the quality and duration of life for some patients with cancer and other serious illnesses, technical expertise alone does not fully account for the upsurge in the societal standing of medical doctors during the past 100 years.
High regard for physicians is contingent on the trust that doctors act unselfishly in a patient’s best interests, a role sometimes called moral fiduciary.2 Essential to this role are medical knowledge and a firm grasp of ethics. Derived from the Greek η´θο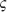 (ethos), meaning character, ethics refers to the process of applying values and principles in professional interactions and particularly in medical decision making on behalf of patients. The curriculum of most medical schools now includes coursework in ethics, but many physicians practicing today completed their training without formal instruction in this topic. To foster an appreciation for the intellectual underpinnings of modern medical ethics, this chapter begins with an overview of scholarly approaches to ethics. Next is a discussion of selected proclamations and codes of ethics published by professional societies, federal commissions, and other authorities as guidelines for medical practice and research. Finally, common ethical issues in radiation oncology are addressed, including relevant medicolegal considerations.
(ethos), meaning character, ethics refers to the process of applying values and principles in professional interactions and particularly in medical decision making on behalf of patients. The curriculum of most medical schools now includes coursework in ethics, but many physicians practicing today completed their training without formal instruction in this topic. To foster an appreciation for the intellectual underpinnings of modern medical ethics, this chapter begins with an overview of scholarly approaches to ethics. Next is a discussion of selected proclamations and codes of ethics published by professional societies, federal commissions, and other authorities as guidelines for medical practice and research. Finally, common ethical issues in radiation oncology are addressed, including relevant medicolegal considerations.
 CONCEPTUAL APPROACHES TO MEDICAL ETHICS
CONCEPTUAL APPROACHES TO MEDICAL ETHICS
The term bioethics, coined in the early 1970s, refers to the academic inquiry and public policy movement addressing the application of science and medicine from a humanistic perspective.3 Medical ethics may be most accurately viewed as a branch of bioethics, but the terms are commonly used interchangeably. Among the more influential theoretical approaches to ethics that are used in bioethics are utilitarianism, deontology, casuistry, virtue theory, and principlism.
Utilitarianism is based on the premise that, in any situation, the best course of action is the one that produces the maximum net positive value (or least negative value). Utilitarianism can be applied to an individual patient’s case or to matters of health policy, in which decisions might be made to achieve the greatest overall benefit for the largest number of people.
Unlike utilitarianism, deontology is an ethical theory based on the morality of actions themselves rather than their net result. Deontology, sometimes called Kantianism in recognition of the influence of Immanuel Kant,4 calls for consistent standards of behavior at all times, regardless of the consequences. One simple example of the difference between utilitarianism and deontology is the issue of educating patients about their diagnosis, especially if the prognosis is very poor. Whereas a deontologist would always feel obliged to tell the truth even at the risk of causing distress, a utilitarian considers whether telling a patient the complete truth about the disease does or does not really benefit the patient. In practice, there is obviously a need to find the right balance between purely utilitarian and purely deontologic perspectives in a situation like this one. Respect for the patient’s autonomy, for example, is one of the prima facie ethical principles discussed further in the next section. Truth-telling is an integral component, and overriding this principle requires justification.
Casuistry is an approach to ethics that emphasizes inductive reasoning based on established precedents, and it is a common practice in both law and medicine. The proper course of action in any individual case is decided by recalling decisions made in prior similar cases. The major weakness of casuistry is the lack of a reference benchmark or settled opinion on novel technologies.
Virtue theory focuses on the character of moral agents, in this case physicians and other health care providers, focusing less on actions or outcomes. As such, virtue theory captures the way in which correct moral actions occur. For example, it is not only important to tell patients the truth about their diagnoses, but it is also critical to do so in a compassionate manner. In this case, the virtue is compassion. Accordingly, while virtue is a critical part of assessing moral action, virtues themselves have little guiding force.
Principlism is a system of applied ethics through which core principles ideally govern behavior in the absence of compelling reasons to override them. Principlism can be integrated with the other philosophical approaches already mentioned and serves as a unifying influence. Key features of principlism are addressed in the next section.
FIGURE 99.1. Early 19th-century engraving depicting a likeness of Hippocrates, closely resembling images of ancient coins found on the island of Cos bearing his likeness. (Courtesy of the National Library of Medicine.)

 ETHICAL PRINCIPLES
ETHICAL PRINCIPLES
A prima facie obligation may be defined as one that is “binding unless overridden or outweighed by competing moral obligations.”4 Four prima facie principles of bioethics are highlighted here: respect for autonomy, beneficence, nonmaleficence, and justice.
Respect for a patient’s autonomy is based on respect for the right to individual liberty. A patient’s voluntary decision to seek medical care or comply with referral to a specialist is the starting point of most patient–physician relationships, and competent patients should remain free to forgo therapy or change physicians at any time. The responsibility to respect patients’ autonomy is established not only in ethics but also in law. An example is the requirement to obtain a patient’s informed consent for proposed medical therapy. In a landmark case involving postmastectomy chest wall radiation therapy, a patient who sustained soft tissue necrosis won a lawsuit against her radiation oncologist for negligence and for lack of proper disclosure regarding possible treatment-related toxicity. The Kansas Supreme Court ruled that doctors should explain “in language as simple as necessary” the side effects associated with any recommended therapy.5
The principle of beneficence is intertwined with that of nonmaleficence. Some authors have offered nuanced distinctions between the two principles,4 but the key point is that physicians should act for the benefit of patients and should not harm their patients. Beneficence and nonmaleficence are typically concordant objectives, but sometimes an intervention that is helpful also entails a high risk of adverse treatment-induced sequelae. For example, administering high-dose morphine to a patient severely dyspneic from an incurable lung malignancy can relieve symptoms but at the same time risks fatally suppressing respiratory drive. Here the quality of remaining life might be improved by a measure that also hastens death. Such an action can sometimes be justified by the principle of double effect, the earliest expression of which is generally attributed to St. Thomas Aquinas.6 According to this concept, the benefit of an action might be valuable enough to outweigh a simultaneous significant risk of serious adverse event, as long as achieving benefit is the primary intent. High-dose chemotherapy, bone marrow transplant, and some high-complexity surgeries, such as Whipple procedures, are examples of cancer therapies in which potential burdens and risks might militate against potential benefits.
The principle of justice refers to fairness, typically the equitable distribution of health care resources. Justice-related questions often arise in regard to matters of public policy. Two examples are determining the means to allocate scarce organs for transplantation and selecting what types of research merit government funding. More globally relevant in the United States is the challenge of allocating federal dollars for health care. Distributive justice implies that persons ought to be afforded medical care according to their medical needs and the ability of the system to provide.
 FORMAL OATHS AND CODES OF ETHICS
FORMAL OATHS AND CODES OF ETHICS
There are many published declarations of medical ethics authored by physicians. Not surprisingly, the tone and language of each reflect the social mores and sometimes historical events of the era in which it was composed.
The Hippocratic Oath
I will prescribe regimen for the good of my patients according to my ability and my judgment and never do harm to anyone
The Corpus Hippocraticum is a collection of medical treatises dating from around the fifth century BCE, believed to be the work of philosopher–physicians from the Greek island of Cos (Fig. 99.1). Contained within the Corpus is the well-known oath of Hippocrates, an ancient physician’s pledge of professionalism. Curiously, the oath is inconsistent with some other sections of the Corpus, perhaps because it was added later. Comments about abortion and surgery, for example, are at variance with teachings elsewhere in the collected works.7 Nevertheless, timeless themes are included, and the oath has survived in various modernized versions. The oath is often recited by medical students at the time of graduation—even if its contents are not always well remembered.8,9
Percival’s Medical Ethics
Hospital physicians and surgeons should minister to the sick, with due impressions of the importance of their office, reflecting that the ease, the health, and the lives of those committed to their charge depend on their skill, attention, and fidelity.
Thomas Percival (1740–1804) (Fig. 99.2) published Medical Ethics at a time when there were considerable tension among clinicians in his community.10 In the 1760s, Manchester, England, was a prosperous urban society that comfortably supported public health initiatives, including an infirmary serving as a charity hospital and teaching institution. But as the city grew and became crowded by the 1790s, tensions arose between rival groups in the medical community. Percival was prompted to draft a code of ethics after a contentious dispute about enlarging the infirmary’s staff, a threat to the controlling physician faction.11
When he began writing Medical Ethics, Percival was already a well-known writer and moralist whose A Father’s Instructions to His Children, published in 1775, included essays promoting personal virtues and social awareness to young readers. While completing Medical Ethics, Percival suffered devastating personal tragedies, bereaving the untimely deaths of two of his own sons. The finished work was dedicated to a third son studying medicine at the time.
Ultimately, Medical Ethics provided a template for the codes of ethics adopted by the American Medical Association (AMA) and other societies in the 19th century. Early 20th-century pundits criticized Percival’s work as merely a book of medical etiquette rather than medical ethics. However, revisionist historians have subsequently argued that although Percival focused on interprofessional relationships, he also taught that a physician’s duty toward the patient outweighs obligations of civility toward other health care professionals. Furthermore, Percival advanced the enlightened view that indigent patients should receive the same quality of care as affluent patients.12
FIGURE 99.2. Thomas Percival (1740–1804). (From Brockbank EM. Sketches of the lives and work of the honorary medical staff of the Manchester Infirmary, from its foundation in 1752 to 1830 when it became the Royal Infirmary. Manchester, UK: Manchester University Press, 1904, with permission.)

The American Medical Association Code of Ethics
The AMA first adopted a code of ethics at its inaugural meeting in 1847. The initial code was modeled on the work of Percival, but subsequent updates have reflected societal changes and technological progress. The 2004 version includes four components13:
a. Principles of Medical Ethics;
b. Fundamental Elements of the Patient–Physician Relationship;
c. Current Opinions of the Council on Ethical and Judicial Affairs; and
d. Reports of the Council on Ethical and Judicial Affairs.
The seven Principles are general instructions for a physician to maintain competence and integrity in the context of individual patient care and also in the larger view toward enhancing the standard of care in the community. The Fundamental Elements expand the concept of a collaborative interaction between physicians and patients, specifically mentioning the need for good communication and the need to protect patients’ confidentiality. The AMA here advocates that all patients have a right to “necessary care,” regardless of their ability to pay for that care, and that physicians should play a role in safeguarding this right. The Current Opinions and the Reports of the Council on Ethical and Judicial Affairs provide situational interpretations of the Principles and Fundamental Elements, and they are often referenced in legal proceedings.
The Nuremberg Code and the Declaration of Geneva
During World War II, odious crimes were committed by Nazi physicians who conducted horrific experiments on concentration camp prisoners who were coerced to submit. The Nuremberg Code was a formal response to these World War II–era human rights atrocities disguised as medical experiments.14 Issued from the military tribunal that tried some of the Nazi doctors who conducted these experiments, the Nuremberg Code acknowledges that medical investigations are important. However, for a medical experiment to be morally permissible, it must meet 10 criteria, paraphrased as follows:
• Voluntary consent of the human subject
• Necessity to yield results helpful to society
• Appropriate design based on knowledge of the disease under study
• Avoidance of unnecessary physical and mental suffering and injury
• Absence of reason to believe that death or disabling injury will occur
• Overall degree of risk in proportion to the nature of the problem to be solved
• Adequate precautions against the possibility of the subject’s injury, disability, or death
• Qualified persons conducting the study
• Unrestricted freedom of the subject to end the experiment if he or she reaches the physical or mental state where continuation of the experiment seems impossible
• Willingness of the investigator to discontinue the study at any time if there is reason to believe that continuing the experiment is likely to result in injury or death to the subject.
In the aftermath of the war, the international medical community was especially sensitized to the need for universal adherence to high standards of ethical behavior. The Declaration of Geneva was adopted in 1948 by the World Medical Association and has been updated since then. The text includes the physician’s vow to ignore “considerations of age, disease or disability, creed, ethnic origin, gender, nationality, political affiliation, race, sexual orientation, or social standing” in the treatment of a patient and to uphold “even under threat … the utmost respect for human life.”
Case Study
Despite publicity about the Nuremberg Code and the Geneva declaration, controversial large-scale, government-sponsored medical studies took place in the years following World War II. Experimentation on the effects of radiation exposure on humans was conducted in the United States during the Cold War, when fears of nuclear warfare prompted inquiry into the carcinogenic and other adverse health effects of environmental exposure to ionizing radiation. In response to public concern about thousands of federally funded studies conducted from the 1940s through 1970s without consent of the subjects, in 1994 President Bill Clinton established the Advisory Committee on Human Radiation Experiments (ACHRE). ACHRE was comprised of ethicists, radiation oncologists, and others with relevant expertise. It was charged with evaluating the experiments’ ethical and scientific standards and recommending actions to ensure that any mistakes of the past would not be repeated. ACHRE reviewed all available documentation and also conducted an oral history project in which scientists described prevailing sentiments regarding human research ethics during the era of interest.
ACHRE found that government officials and investigators were in some cases culpable “for not having had policies and practices in place to protect the rights and interests of human subjects who … could not possibly derive direct medical benefit.”15 One example was the observational study of uranium mine workers exposed to radon levels known to be hazardous, without warning and without efforts to reduce the radon levels by ventilating the mines. As a result, lung cancer developed in hundreds of workers, and appropriate compensation was recommended for the individuals affected.
The Belmont Report
In 1972 Jean Heller exposed the injustices of the U.S. Public Health Services (USPHS) Study of Untreated Syphilis in the Negro Male that was conducted in Tuskegee, Alabama.16 During a 40-year period beginning in the 1930s, 399 indigent African American sharecroppers with syphilis and 200 without syphilis were subjects in a natural history study of the disease. The men mistakenly believed that diagnostic blood tests and lumbar punctures composed treatment for their “bad blood” when in reality these were done solely to monitor the course of the infection. Moreover, years later when penicillin was discovered and found to be effective in the treatment of syphilis, it was withheld intentionally from the men. Subsequently, public awareness of the USPHS study likely contributed to enduring reluctance among some members of minority groups to participate in clinical trials,17,18 and the political backlash provoked action by the federal government.
The 1974 National Research Act established the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The commission met at the Belmont Conference Center in Maryland to develop ethical guidelines for the conduct of biomedical and behavioral research. In 1979 the commission published Ethical Principles and Guidelines for the Protection of Human Subjects of Research, commonly known as the Belmont Report.19
In the Belmont Report, a clear distinction is made between medical research and clinical practice. The term practice describes interventions intended “to enhance the well-being of an individual patient … that have a reasonable expectation of success,” whereas research is “designed to test an hypothesis, permit conclusions to be drawn, and … contribute to generalizable knowledge.” Sometimes a clinician uses good judgment and departs from standard methods for the benefit of an individual patient in special circumstances. However, if major innovations are proposed as replacement for standard techniques, then a formal investigation should be conducted to assess safety and efficacy. The National Commission embraced a principlist perspective in drafting the report, emphasizing in particular respect for persons, beneficence, and justice. The Belmont Report’s definition of respect for persons incorporates respect for autonomy and special concern for individuals with diminished capacity to exercise their autonomy. Involving prisoners in research activity is cited as an example. Although it is inappropriate to deny prisoners the possible benefit of experimental interventions, any direct or indirect pressure on prisoners to participate in clinical studies must be avoided. For instance, a promise of clemency in return for study enrollment is unacceptable. The report also addresses the nature of justice in medical research, emphasizing that the process of selecting subjects for a research study must be carefully examined. Investigators should minimize the chance that socioeconomically disadvantaged groups are represented disproportionately as a result of a vulnerability to manipulation in the health care environment.
The American College of Radiation Oncology Code of Ethics
The American College of Radiation Oncology (ACRO) has published a code of ethics, contained within the organization’s bylaws, which is available for review at the organization’s website (www.acro.org). The principles expressed are concordant with accepted ethical standards and include respect of patient autonomy, the expectation that a radiation oncologist should always act in the best interests of the patient, and respect of patient confidentiality. The ACRO code also forbids deceptive billing arrangements, and members of ACRO who do not comply with the code of ethics are subject to disciplinary action by the organization.
National Electrical Manufacturers Association Code of Ethics
The makers of equipment and software used in the practice of radiation oncology are not required to demonstrate clinical efficacy of a new device or software through the same sort of clinical testing that is required by the U.S. Food and Drug Administration (FDA) for approval of a new drug or implantable medical device. Rather, most treatment devices are approved after demonstration of safety and substantial equivalence to an approved device that is already commercially available. A company that wishes to sell a new device submits a premarket notification to the FDA at least 90 days before commercial distribution is to begin, in accordance with Section 510(k) of the FDA Modernization Act of 1997. Because devices may be introduced into the market without proof of superiority in any given clinical situation, manufacturers may promote their products to physicians by emphasizing intuitively attractive features that might or might not provide meaningful clinical advantage to patients.
On January 1, 2005, members of the National Electrical Manufacturers Association (NEMA) adopted a code of ethics regarding interactions between makers of medical imaging and treatment equipment and physicians (www.nema.org). Individual sections of the NEMA code address member-sponsored product training and education, support for third-party educational conferences, sales and promotional meetings, arrangements with consultants, gifts, provision of reimbursement and other economic information, charitable donations, and research grants. The guidelines are essentially consistent with the AMA policy on gifts and applicable federal regulations. NEMA members are allowed to support educational conferences and advertise their wares in these venues, and also they may provide educational support for individual customers in the safe use of their products. However, hospitality provided by NEMA member at conferences and meeting should be “modest in value and … subordinate in time and focus to the purpose of the meeting.” When it is necessary to demonstrate nonportable equipment, members may pay for reasonable travel costs of attendees with a bona fide professional interest but not for their guests.20
 COMMON ETHICAL ISSUES IN RADIATION ONCOLOGY
COMMON ETHICAL ISSUES IN RADIATION ONCOLOGY
Doctors wear a lot of hats these days; besides the usual physician role, we are expected to be a researcher, financial counselor, administrator, gatekeeper, patient advocate in the medicolegal system, ethicist, and Lord knows what else.
—Thomas J. Smith21




