Major Concepts
Disease
Infection with the enterohemorrhagic O157:H7 strain of Escherichia coli may lead to mild, self-resolving intestinal illness (in the majority of cases) or life-threatening conditions such as hemorrhagic colitis, hemolytic uremic syndrome (HUS), and thrombotic thrombocytopenic purpura. HUS is most common in infants, the elderly, and immunosuppressed persons, and antibiotic use appears to increase the risk of developing this disease manifestation. The first reported outbreaks with O157:H7 occurred in 1982 in the American Pacific Northwest and later in Michigan. Large and small outbreaks have been reported in many other areas of the world since that time. Disease severity, as assessed by frequency of hospitalizations and HUS incidence, is increasing as infection with the more pathogenic clade 8 becomes more prevalent.
Infection
O157:H7 is a pathogenic strain of E. coli, a species of gram-negative cocci. The bacteria contain genes for several virulence factors that increase their ability to survive and disseminate under the diverse conditions to which they are exposed as they pass through the guts of mammals and into the external environment. These virulence factors include the Shiga toxins, proteins encoded by the LEE genes, enterohemolysin, EspP, catalase peroxidase, and an iron-transporting protein. These are found on the bacterial chromosome, a plasmid, and a bacteriophage.
Transmission
The O157:H7 strain is carried in the digestive tract of normal healthy ruminants, such as cattle and sheep, and is shed into the external environment during defecation. Animals from different feedlot pens may have very different prevalences of infection, as reflected in the wide-ranging percentages of infectious feces and contaminated hides found in the different pens. Both materials contribute to infection of meat during processing. If the external surface of the meat becomes infected and the meat is subsequently ground, bacteria are spread to the internal portions. Ground meat therefore needs to be prepared in a manner that thoroughly cooks all regions. Refrigeration, freezing, and conditions of low pH will not kill the majority of these bacteria. In addition to the infected meat itself, materials and surfaces coming into contact with uncooked meat may spread the bacteria onto other food items, some of which are consumed raw. Raw milk, tap water, vegetables, fruits, and fruit juices may also become infected by contact with manure used in fertilization or water contaminated with manure. Human-to-human transmission may occur when fecal material from an ill person comes into contact with food or with objects in day care centers or in health care settings. Infections may also result from swimming in contaminated, unchlorinated water.
Protection
Treatment for E. coli O157:H7 infections is typically supportive and involves balancing fluid and electrolyte levels, and for more severe cases, blood transfusions, renal dialysis, or both. Antibiotic use is not recommended, as killing the bacteria releases toxins and increases the risk of developing HUS. Several vaccine candidates have been tested in cattle, with poor results.
Escherichia coli is a common commensal bacterium found in the guts of humans and some other mammals, including cattle and sheep. Though normally beneficial for their human hosts, some strains of E. coli cause serious disease. Pathogenic groups of E. coli include enterohemorrhagic strains, such as O157:H7. Presently as many as 30 outbreaks with that strain alone occur in the United States each year, during which about 20,000 people become ill and approximately 250 die. Transmission often occurs by eating or drinking food or liquid tainted with fecal material. The sources of contamination range widely and include hamburgers, alfalfa sprouts, yogurt, fruit juices, and tap water. Humans are infected by other materials as well. Day care centers may allow transmission of E. coli between young children as toys and other objects come into contact with fecal material and the bacteria are transferred to fingers and then to food items or directly to the mouth. People have also been infected in public pools from ill infants placed into the water without proper swimming diapers.
Although the general public is aware of the dangers of eating undercooked hamburgers and of the importance of properly washing one’s hands after using the restroom or changing diapers, particularly for those employed in the food industry or in the care of children or patients, compliance is not complete. Due to greater public awareness and governmental regulation of restaurants, the overall numbers of infections with O157:H7 strains have been decreasing; however, several disturbing trends have also been noted. First, bacterial transmission is continuing to occur through contaminated fruits and unpasteurized fruit juices and vegetables, particularly those consumed raw in salads, such as lettuce, spinach, and tomatoes. Second, the bacterial population appears to be shifting in favor of more pathogenic strains of O157:H7 clade 8, which have increased expression of toxin genes. Progress has been made, however, in detecting conditions that may lead to bacterial survival and transmission in cattle and sheep and may decrease the rate of infection and production of infectious feces by these hosts. Similarly, factors are being identified that increase bacterial persistence on lettuce and other produce.
E. coli was discovered by Theodore von Escherich in 1885. Outbreaks of enterohemorrhagic E. coli (EHEC), especially the O157:H7 strain, often occur through contamination of food or water supplies. These outbreaks have occasionally been quite large and have infected large numbers of people before the source of infection was discovered. In the first such reported outbreak, transmission was found to have occurred through hamburgers served by the popular Jack-in-the-Box fast-food restaurant chain in the Pacific Northwest in 1982. During this outbreak, four children died and 732 people became ill. Another outbreak occurred that year from tainted hamburgers in Michigan. A high proportion of infected individuals were hospitalized (70%), but none of them developed one of the most dangerous manifestations of E. coli infection, hemolytic uremic syndrome (HUS). An E. coli outbreak in Alpine, Wyoming, resulted from the presence of the O157:H7 strain in area tap water. The water did not taste or smell any differently from uncontaminated water but caused a sometimes serious infection. A very large outbreak due to contaminated food occurred in Japan in August 1996. During that outbreak, 6,309 children and 92 staff members from 62 municipal schools were infected with O157:H7 obtained from radish sprouts. Hundreds of persons were hospitalized, and approximately 100 developed HUS. Canada was struck by a large outbreak involving contaminated municipal water supplies in Walkerton, Ontario, in May 2000. Seven people died and another 2,500 became ill, making this episode one of Canada’s worst public health emergencies. In the United States between 1982 and 2002, some 350 outbreaks of O157:H7 infection occurred, resulting in illness in more than 8,500 persons, 17% of whom were hospitalized and 4% of whom developed HUS. Two U.S. outbreaks in 2006 resulted from consumption of contaminated spinach or lettuce; 275 persons were infected, 51% to 75% required hospitalization, and 11% to 15% developed HUS. A trend toward higher incidence of HUS appears to be developing. This trend may be due to a shift in bacterial populations in favor of more virulent subpopulations.
Infection may also occur between infants, through feces in diapers coming into contact with objects that other infants place in their mouths and may be transferred to their fingers and then contaminate food. In one day care center in Washington, seven children became ill playing with sand, soil, modeling clay, and toys that were soiled with infected feces. Infection may also occur in public pools if ill infants are placed into the water without proper swimming diapers.
Hemorrhagic Colitis
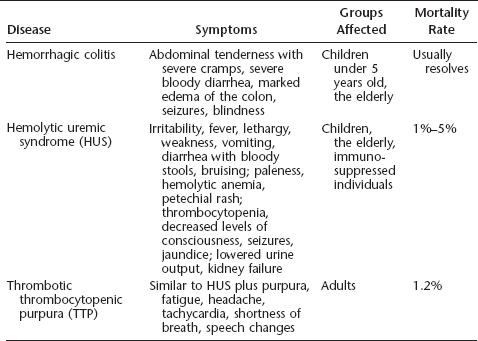
Infection with as few as 10 viable organisms of EHEC strains may result in a variety of symptoms ranging from asymptomatic or mild (primarily diarrhea) to severe and life-threatening. The incubation period is one to six days. Disease manifestations include vomiting and nausea, abdominal tenderness and severe cramps, and diarrhea, which in 35% to 90% of infected individuals becomes bloody after two to three days (the syndrome is known as hemorrhagic colitis). Another symptom of hemorrhagic colitis is the marked edema of portions of the colon in the absence of other stool pathogens. Symptoms do not improve if one does not eat. If a fever is present, it is low-grade. The lack of significant fever together with abdominal tenderness may suggest a variety of noninfectious causes, including inflammatory bowel disease, appendicitis, ischemic or ulcerative colitis, diverticulitis, and Crohn’s disease. Neurological symptoms, including seizures and blindness, may also be present. Symptoms may also be confused with those caused by other infectious microorganisms, such as Campylobacter, Clostridium difficile, Entamoeba histolytica, Salmonella, Shigella, and Yersinia enterocolitica. Some of the virulence genes from O157:H7 bear a great degree of homology with those found in several of these other bacteria and may in fact have been acquired from them via motile genetic elements, such as plasmids and bacteriophages. Symptoms usually resolve within a week; however, some individuals develop more serious or life-threatening infections. The chance for developing severe disease is greatest in children under the age of 5 years and the elderly.
Hemolytic Uremic Syndrome (HUS)
In addition to hemorrhagic colitis, the young and the elderly also have an increased susceptibility to HUS. Early symptoms of HUS include irritability, fever, lethargy, weakness, vomiting and diarrhea, and bloody stools. Later signs may include bruising; paleness due to extensive lysis of red blood cells (hemolytic anemia); petechial rash; decreased platelet numbers (thrombocytopenia); decreased levels of consciousness, seizures, or other central nervous symptoms; jaundice; and lowered urine output with or without kidney failure (in 2% to 7% of cases). Over 85% of the cases of postdiarrheal HUS are believed to be due to infection with O157:H7, and the majority of the remaining cases are attributed to infection with other E. coli strains that produce Shiga toxins (described later in this chapter). The overall mortality rate is 1% to 5%, and deaths occur primarily in children, the elderly, and immunosuppressed individuals. The fatality rate for this syndrome among children is 3% to 5%. Those who survive have an increased incidence of high blood pressure or kidney impairment (or both) later in life.
Thrombotic Thrombocytopenic Purpura (TTP)
Another serious result of O157:H7 infection is thrombotic thrombocytopenic purpura (TTP). It includes many of the symptoms listed for HUS, but with less prominent neurological manifestations and diminished renal damage. It additionally includes bleeding under the skin, resulting in the formation of purple-colored spots (purpura), fatigue, headache, tachycardia (elevated heart rate), shortness of breath, and speech changes. TTP is also more often reported in adults and is usually not preceded by diarrhea. In some of the early outbreaks in the United States, the hospitalization rate was 23%, HUS or TTP developed in 6%, and death occurred in 1.2% of the cases; not all of the fatalities were related to either HUS or TTP.
Table 7.1 Diseases associated with E. coli O157:H7 infection
General Characteristics of E. coli O157:H7
E. coli bacteria are typically harmless commensal gram-negative rods that reside in two very different types of environments: (1) in the rumen or the large or small intestine of humans and some other mammals and (2) in the external environment, with its limited nutrients and variable temperature and water availability. They are usually beneficial to their mammalian hosts and help metabolize food in the digestive tract. Several strains of E. coli, however, cause disease in humans. Some strains of these bacteria, such as the EHEC group, are pathogenic; one such strain is O157:H7, which may cause severe or fatal illness. Infection with other EHEC strains, including O26:H11, O111:H8, and members of the O103 and O118 serogroups, are becoming emerging problems and appear to be more common than O157 strains in some regions. “Montezuma’s revenge,” a bane of travelers, is due to infection with EHEC strains. Other major groups of pathogenic E. coli strains are the enteroaggressive strains (which cause persistent diarrhea in very young children from developing countries), enteropathogenic (EPEC) strains (infantile diarrhea), enteroinvasive strains (watery diarrhea), enterotoxogenic strains (traveler’s diarrhea), and diffuse adherent strains (diarrhea in preschool-aged children).
FIGURE 7.1 Scanning electron micrograph of E. coli O157:H7
Source: CDC/National Escherichia, Shigella, Vibrio Reference Unit.
Phylogenetic analysis of over 500 clinical isolates of the O157:H7 strain have allowed it to be divided into nine distinct clades. Of these, infection with clade 8 bacteria is associated with the highest risk of developing HUS. This clade has become increasingly prevalent since 2003, and a particularly virulent subpopulation of this clade is evolving. Changing O157:H7 population dynamics may thus be at least partly responsible for the recent increased pathology in infected humans. O157:H7 strains differ from commensal E. coli strains in their ability to ferment sorbitol and dulcitol and to oxidize other carbon sources. They contain over 1,600 genes that are not found in commensal strains; the vast majority of these are not virulence factors but may allow the bacteria to outcompete other strains for limiting nutrients.
E. coli, like other gram-negative bacteria, contain lipopolysaccharide (LPS) in their cell walls. Strains of these bacteria are defined by their somatic (O) and flagellar (H) antigens. Differences in the LPS O-antigen have been found in different diseases of humans and animals. The O157 antigen may be involved in virulence (discussed later in this chapter). H7 specifies the type of flagellin protein and is encoded by the fliC gene. It has been hypothesized that O157:H7 may be derived from the EPEC strain O55:H7, which is able to adhere tightly to epithelium in the gut. This strain is believed to have acquired the fliC gene for H7 flagellin, followed by the Stx2 toxin and EHEC plasmid (to be discussed shortly). The gene for the Stx1 toxin was a later addition, and the ability to ferment sorbitol and β-glucuronidase activity was lost during evolution of the EHEC O157:H7 strain that is commonly found throughout North America and Europe. It is closely related to the O157:H- strain, which causes hemolytic uremic syndrome in Bavaria in Germany. That strain lacks the H7 flagellin and Stx1 but has β-glucuronidase activity and can ferment sorbitol.
O157:H7 bacteria prefer to grow at human body temperature, 37°C (98.6°F), but may grow at a wide range of temperatures from 10°C to 50°C, allowing them to withstand many conditions that they may encounter in the external environment before infecting a suitable host. The O157:H7 strain is also better able to survive and reproduce in acidic conditions than nonpathogic strains of E. coli, including food with a pH as low as 4.4. This acid resistance allows the majority of these bacteria to survive passage through the stomach. Acid tolerance is increased at low temperatures, as in refrigerated salad dressing or apple juice.
Bacterial Reservoirs and Transmission
E. coli normally resides, in addition to humans, in the rumen and terminal colons of both ill and healthy cattle and sheep, which serve as disease reservoirs. Examination of healthy cattle in the United Kingdom found that the O157:H7 strain could be isolated in 0.9% to 15.7% of the animals. Calves are more commonly infected than adult cattle and appear more susceptible to developing disease. Raw milk from infected dairy cows may transmit disease. Transmission through the fecal route may be seasonal, as infected sheep from Idaho did not shed virus via defecation in November, but many (approximately 30%) did so in the warmer month of June.
RECENT DEVELOPMENTS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree