Fig. 18.1
Scanning electron microscopy (SEM) micrographs showing the morphology of erionite fibers, crocidolite asbestos, and chrysotile fibers. (a) Erionite f ibers from Pine Valley, NV (courtesy of Dr. Ian Steele, Department of Geoscience, University of Chicago, Chicago, IL). (b) NIEHS reference sample of crocidolite asbestos. (c) A bundle of chrysotile fibers (arrow) interacting with the tracheal epithelial cell surface in organ culture (from [86]).
Erionite has gas adsorption, ion exchange, and catalytic properties that are highly selective depending upon the molecular size of the adsorbed compounds [6]. The integral composition of erionite is made up of an interlocking tetrahedral of silicate aluminate which is negatively charged, allowing it to bind cations [7]. In addition, erionite adsorbs iron after inhalation and interacts with the respiratory epithelium. This adsorbed iron is associated with the toxicity of erionite [8].
Three types of erionite have been identified based upon differences in their levels of Ca, Na, and K (the predominant cations) and the amounts of Mg, Fe, Sr, and Ba in the structure. The general formula for the erionite-Ca type mineral is (Ca3.56K1.95 Na0.27Mg0.30) (Si25.78 Al10.28 Fe0.01) O78, for the erionite-Na type mineral is (Na4.00 K2.40 Ca1.13Mg0.24) (Si26.69 A19.11Fe0.22) O72, and for the erionite-K type mineral is (K2.80Na1.66Ca1.03Mg0.51) (Si28.21 A17.39 Fe0.41) O72 [9].
Asbestos is a commercial term for another group of naturally occurring crystalline fibers. Each group has its own distinctive structure and chemical composition. There are two subgroups: (1) a serpentine group, consisting of chrysotile [Mg6Si4O10 (OH)8]; and (2) the amphiboles, five groups of rod-like fibers including crocidolite [Na2(Fe3+)2(Fe2+)3 Si8O22(OH)2], amosite [(Fe,Mg)7Si8O22(OH)2], tremolite [Ca2Mg5Si8O22(OH)2], anthophyllite [(Mg,Fe)7 Si8O22(OH)2], and actinolite [Ca2(Mg,Fe)5Si8O22(OH)2] [10]. These fibers may exist in deposits with each other or other minerals and may adsorb other metals or organic compounds to their surfaces during mining or processing.
The potential of asbestos fibers to cause cancers and fibrosis has been linked primarily to their fibrous geometry and size—longer, thinner fibers being most bioreactive because of their incomplete phagocytosis, release of oxidants from cells, and their ability to act as a platform for cell proliferation [11, 12]. However, in addition to size, the chemical composition of asbestos fibers also plays an important role in determining the cytotoxicity, durability, and biopersistence of asbestos types [13]. Amphibole fibers, especially crocidolite and amosite asbestos, have the greatest carcinogenic potential in MMs in comparison to chrysotile in asbestos-exposed human populations. The increased pathogenicity of amosite and crocidolite asbestos may reflect their greater durability in the lung or pleura after inhalation as well as the high iron content of these amphiboles (20–30 % by weight) [13]. They also form ferruginous asbestos bodies in the lung in contrast to chrysotile fibers which may be iron-free or acquire iron naturally in trace amounts. Amphibole asbestos fibers also have a sharp, needle-like structure which causes their alignment with airways after inhalation and allows more effective penetration into the deep lung. In contrast, chrysotile fibers have a curly, snake-like structure (Fig. 18.1c) and break down into smaller fibrils over time. A number of studies have shown that smaller fibers or fragments (<5 μm) of asbestos, regardless of type, are less cytotoxic, carcinogenic, and fibrogenic than longer asbestos fibers [14]. This may be in part because they are more easily cleared from the lung over the long latency periods of asbestos-induced diseases.
On an equal weight basis, chrysotile fibers are more cytotoxic than amphibole fibers due primarily to their positive surface charge and greater surface area whereas amphiboles are neutral or slightly negative in charge [15]. Hydrated magnesium molecules are located on the apical oxygens of chrysotile accounting for its highly polar properties as well as dissolution of the mineral when magnesium is leached from the fiber. In nature, these structures are arranged as loose bundles that break down into smaller fibrils in tissue [1, 16].
For reasons that are unclear, erionite is more carcinogenic in rodents than asbestos, regardless of source [7]. Experimental studies indicate that erionite is 300–800 times more carcinogenic than chrysotile and 100–500 times more carcinogenic than crocidolite asbestos when administered through intrapleural routes. In intraperitoneal injection studies, erionite is 20–40 times more potent than chrysotile, and 7–20 times more potent than crocidolite in tumor induction [17]. These trends have also been observed in inhalation studies [18]. One theory is that erionite has a hexagonal structure with an internal surface of 200 m2/g that is 20 times larger than crocidolite asbestos, thus allowing erionite to absorb many small molecules and to have a higher catalytic activity [19]. It was also found that on an equal weight basis, erionite induces more reactive oxygen species (ROS) than asbestos fibers [20].
18.3 Links Between Erionite, Amphibole Asbestos, and Malignant Mesothelioma
18.3.1 Features of Malignant Mesothelioma
Malignant mesothelioma (MM) is an aggressive tumor originating from the lining cells (mesothelium) of the pleural and peritoneal cavities, as well as the pericardium and the tunica vaginalis. Although the main risk factor is e xposure to asbestos or erionite, other factors such as Simian virus 40 (SV40) infection and inheritance of susceptibility genes likely play roles in initiation and progression of MM [21, 22]. MM may acquire many phenotypic forms (epithelioid, biphasic, and sarcomatoid) which makes them difficult to diagnose without multi-panel histochemical stains.
The combination of an accurate patient history, physical examination, radiology, and the confirmation of pathology is essential in the diagnosis of mesothelioma. Typical presenting features invariably occur in late stages of disease and include chest pain, dyspnea, and breathlessness due to pleural effusion [23, 24]. Treatment strategies include chemotherapy, radiotherapy, gene therapy, extrapleural pneumonectomy, surgical resection of tumors, or combined therapies. However, no effective therapies have been developed for MM, and because they are most often diagnosed at end stages of disease, clinicians often focus on palliation of symptoms and/or end-of-life care [25].
The latency period for development of MM is often 30–40+ years from initial exposure to asbestos fibers [13]. Because of difficulties in treatment and management, life expectancy in pleural MM is grim with median survival varying between 8 and 14 months [21]. However, peritoneal MM patients can survive 5 years or longer [26].
18.3.2 Malignant Mesothelioma in Turkey
18.3.2.1 Epidemiologic Studies
Annual asbestos-related mesothelioma cases in Southeast Turkey are reported to be 50 per million [27]. However, the annual incidence of mesothelioma related to e nvironmental exposure to erionite was found to be from 2200 to 8000 cases per million in several villages such as Karain, Tuzkoy, and Sarihidir in the Cappadocia region of Turkey. This incidence rate is much higher than any other reported incidence of MM worldwide [28]. In 1987, Baris reported the epidemic of mesothelioma among residents of Karain, Tuzkoy, and Sarihidir [28]. The estimated incidence of pleural MM was indicated as 996 per 100,000 inhabitants [28]. It was noted that more than 50 % of deaths were caused by MM in these three villages [29]. A study based on 162 Turkish immigrants from Karain to Sweden also shows a strong correlation between erionite exposure and MM. Fourteen deaths were reported due to MM among the 18 (78 %) total deaths during the period between 1965 and 1997. The standardized incidence rates found in this study were 135 times higher among the males and 1336 times higher among the females compared to the general population of Sweden [30].
A mortality study between 1994 and 1997 suggested that MM might also occur in some of the other villages near the known three erionite villages in Cappadocia. These other villages are Cokek, Karlik, Karacaoren, Boyali, and Yesiloz. These investigators reported 53 deaths due to mesothelioma from the three erionite villages, Karain, Tuzkoy, and Sarihidir, and 11 deaths from the other four villages [31]. It was noted that MM cases were more prevalent in the erionite villages compared to the nearby villages despite the environmental contamination of erionite, which was often used in dwellings in the region [27].
In 2006, a study from 1979 to 2003 based on residents of two erionite-exposed, and one nearby control village, was published [32]. In this study, a total of 891 men and women, aged 20 years or older, were included, a total of 230 residents in villages without known exposure to erionite. Mortality data were obtained from hospital records and death certificates. During the 23-year follow-up, 119 deaths occurred from MM, the cause of 44.5 % of all deaths in the exposed villages. Seventeen patients had peritoneal mesothelioma; the remainder died of pleural mesothelioma. Only two cases of MM, one of each type, occurred in the control village, both in women born elsewhere [32].
18.3.2.2 Gene: Environmental Interactions
In Karain, Tuzkoy, and Sarihidir, MM occurs only in certain houses. Pedigree analyses revealed that in MM villages, MM was more frequent in certain families compared with others. However, in some families, MM developed among individuals who married into the affected family. For example, in one family, 17 of 30 members died of MM. Among the 17 MM, 5 occurred in individuals who married into this family [2]. This finding initially argued against a theory of genetic predisposition. However, it was found that those who married into a MM family and developed MM were also from MM families. When members of a family with high incidence of MM married into families with no history of MM, descendants developed MM [2].
To test the hypothesis that genetics alone could cause MM in certain families, 24 descendants from one family (ages 26–45 years) born and raised outside the village of Sarihidir were identified. No MM developed in this group. Instead, three MM cases were observed in the same age group among 29 members of a family born and raised in Sarihidir and exposed to erionite. These pedigree studies indicate that the MM epidemic in three Cappadocian villages is caused by genetic predisposition and may make certain individuals more susceptible to the carcinogenic effects of erionite exposures [2]. A team of US and Turkish investigators is now attempting to isolate the putative MM susceptibility gene(s). This gene(s) may be mutated or otherwise altered in Cappadocian families and also may be affected in sporadic familial MMs in the Western world [32].
18.3.3 Epidemiology of Asbestos-Induced Malignant Mesothelioma
18.3.3.1 Historical Studies
Although adverse health effects of asbestos were first recognized by 1899 [33], dust control legislation for mines was not enacted in North America until 1971 by the Occupational Safety and Health Administration (OSHA). In 1935, the suggestion that mesothelioma resulted from occupational exposure to asbestos was first made by Gloyne [34], and the first clear evidence of a link between amphibole asbestos exposure and primary malignant tumors of the mesothelium was shown by Wagner in 1960 [35]. Wagner’s observation was based on 33 cases of pleural mesothelioma in the Northwest Cape Province of South Africa, 28 in persons who had lived close to the crocidolite mines, mostly as children [35]. Confirmation was provided by eight case-control studies published in 1965–1975, based on a total of 657 cases, predominantly male, exposed to asbestos mainly in shipyards, heating trades, insulation, and factory work [36]. It was also shown that the hazards of asbestos dust were not confined to heavily exposed workers in asbestos factories but extended to insulation workers, other users of products containing asbestos, and people who lived close to asbestos factories [37–40].
The role of SV40, a polyomavirus, as a cofactor with asbestos fibers in the induction of malignant mesothelioma is controversial. A review by Gazdar indicates that some investigators have detected SV40 viral DNA sequences and oncoproteins in human pleural MMs [41]. Even though there are technical concerns about some of these findings [42], a role for SV40 as a carcinogen or co-carcinogen is biologically believable on much cellular data and results of animal models [43]. This suggestion is also supported by many of the molecular mechanisms of action of these viral oncoproteins [41].
18.3.3.2 Libby, Montana
Although approximately 2500 cases of MM are reported in the USA annually, a cluster has been noted in Libby, MT, a town with a population of ~2700, with nearly 12,000 people in the surrounding area. Libby had one of the world’s largest vermiculite mines in operation from the 1920s to 1990. The Libby vermiculite had useful insulating and soi l conditioning properties. However, ore from the mine was contaminated with fibrous and nonasbestiform amphiboles in veins throughout the deposit [44]. After 70 years of mining amphibole-contaminated vermiculite, amphibole asbestos related contamination was seen in areas surrounding the abandoned mine and in other areas throughout the town. For example, tree bark samples contain varying levels of amphibole contamination in Libby in the EPA-restricted mine area [45]. Amphibole fiber concentrations range from 41 to 530 million fibers per gram of bark while a bark sample collected ~11 km west of town along the railroad line had concentrations of 19 million fibers per gram [46]. This indicates that there is a potential exposure to asbestos during harvesting or disturbing contaminated woods in the Libby area. Libby was added to the Environmental Protection Agency’s (EPA) National Priorities List in October 2002 for Superfund sites [47].
It is reported that nearly one million homes in the USA have used vermiculite-based insulation from the Libby mine [48]. Even though vermiculite is not classified as one of the six types of asbestos, Libby amphibole has been described as a tremolite-containing transition fiber, and exposure of residents and past workers at the Libby vermiculite mine has been associated with the development of pleural plaques and numerous asbestos-related diseases including asbestosis, pleural fibrosis, and MMs [49]. In this region, asbestos-related deaths are reported to be 40–80 times greater than reference populations in the USA [49]. Thus, Libby amphibole fibers may also be potent in the pathogenesis of MMs.
18.4 Mechanisms of Action of Asbestos and Erionite in the Development of Malignant Mesothelioma
18.4.1 Modern Concepts of Carcinogenesis
Several decades ago, it was thought that all carcinogens were mutagens, and a number of cell assays, including those using bacteria (the Ame’s test ), were developed with the hopes of identifying critical mutations and short-term testing of agents for carcinogenicity in lieu of expensive lifetime animal studies. Subsequently, it beca me clear in 2000 that multiple, sometimes common, mutations (i.e., in oncogenes) were required for the development of most cancers [50]. It also had become apparent that several of these mutations were spontaneously induced during aberrant cell replication when DNA repair was compromised, causing DNA damage and genome instability [51]. Carcinogens causing mutations in vitro by direct interaction with DNA or after metabolism and formation of DNA adducts are often termed initiators of cancer, although it is clear that genomic instability also occurs during the long latency periods (progression) of most cancers. Genetic instability is augmented by tumor promoters that induce cell proliferation and inflammation, chronic stimuli necessary for the development of cancers. In lung cancers, it is clear that asbestos fibers function primarily as co-carcinogens and tumor promoters, as mutations by asbestos are not observed in human bronchial epithelial cells or other epithelial cells in general [52]. In this scenario, polycyclic aromatic hydrocarbons (PAH) associated with smoking classically cause DNA adduct formation whereas asbestos fibers act as co-carcinogens to adsorb PAH and increase their delivery to lung epithelial cells [53]. On the other hand, asbestos fibers act on tracheal epithelial and mesothelial cells as tumor promoters (analogous to phorbol esters in mouse skin) in that they induce inflammation and IL-1B dependent cytokine production that is triggered by activation of the inflammasome [54]. Chronic cell proliferation is triggered by activation of growth factor receptors and protein kinases, increased cyclin D1 and induction of cycling cells [55], stimulation of ornithine decarboxylase [56], and hydrolysis of phosphatidylinositol, increases in diacylglycerol, and activation of protein kinase C at the plasma membrane [57–59]. Moreover, asbestos increases numbers of tumors at low, nontumorigenic concentrations of PAH in tracheal graft models [60]. None of these alterations are observed using nonfibrous, chemically similar analogs of asbestos. Moreover, longer fibers cause more striking alterations than shorter fibers or fragments.
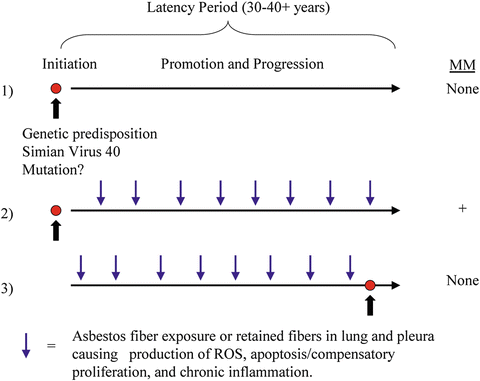
Figure 18.2 depicts a hypothetical model of how amphibole asbestos fibers might promote the development of MMs initiated by tumor susceptibility gene alteration(s) or interaction with SV40, a DNA virus. This schema is supported by studies showing that mutations by asbestos fibers in vitro are large scale chromosomal deletions observed only at toxic amounts of fibers and dissimilar to clonal mutations detected in MM.