Epstein-Barr virus (EBV) is a human γ-herpesvirus that is associated with important syndromes in both the immunocompetent and immunocompromised host. It is a ubiquitous virus, as it is found worldwide. In community settings, EBV is primarily transmitted by exposure to human saliva since humans are the only known host of the virus. Peaks of primary infection occur in early childhood and young adulthood, with the highest rates in persons 10 to 25 years of age. In nonindustrialized countries, 90% of children are infected before age 5, where infection is typically asymptomatic. In industrialized countries, this level of seropositivity is not attained until the fourth decade of life. Although many episodes of EBV infection may be asymptomatic or associated with mild, nonspecific respiratory symptoms, it classically presents with the mononucleosis syndrome with rare progression to more severe disease (e.g., EBV-driven hemophagocytic lymphohistiocytosis [HLH]) in the immunocompetent individual. In contrast, the spectrum of EBV-driven disease broadens to include lymphoproliferative syndromes, including malignancies among individuals with congenital or acquired immune deficiencies, particularly those associated with impairment of cell-mediated immunity. In this chapter, we discuss EBV infection and its complications in children undergoing solid organ transplantation (SOT), hematopoietic stem cell transplantation (HSCT), and those being treated for cancer. EBV-driven primary cancers (e.g., Burkitt lymphoma) are not discussed.
Epidemiology and risk factors
Epstein-Barr virus in solid organ transplant recipients
In the SOT setting, donor-transmitted EBV infection is extremely common in EBV-mismatched (donor-seropositive/recipient-seronegative [D + /R − ]) patients. Transmission is also possible when non-leukoreduced blood products are used. The EBV genome is found in the majority (>90%) of B-cell posttransplant lymphoproliferative disorders (PTLDs) occurring within the first year after SOT. However, PTLD occurring later after transplant is frequently EBV-negative. The highest rate of PTLDs in the SOT setting is seen in the first year after transplant, typically among individuals who are EBV-seronegative recipients of seropositive donor organs.
Primary EBV infection is a major risk factor for PTLD in SOT recipients; therefore pediatric populations are at much a higher risk of developing PTLD than their adult counterparts. Individuals who are R + are not devoid of PTLD risk and account for up to 25% of PTLD cases in children. This may be due to reactivation of latent EBV in the recipient or acquisition of a new strain of EBV. Beyond the specific role of EBV serostatus, the incidence of PTLD varies according to several factors including the type of organ transplanted. Risk related to the latter may reflect immunosuppressive regimens, lymphoid load in the allograft and chronic antigenic stimulation resulting from direct communication and exposure to environmental antigens, or chronic allograft dysfunction including antibody-mediated rejection. Small intestine transplant recipients are at the highest risk for development of PTLD (up to 32%), whereas recipients of pancreas, heart, lung, and liver transplants are at moderate risk (3% to 12%). Renal transplant recipients are at relatively low risk (1% to 2%). Although PTLD lesions in SOT recipients are most often of recipient origin (as this relates to the source of the proliferating cells), lesions that are limited to the graft occurring early after transplant are predominantly donor in origin.
Antilymphocyte globulins that result in selective T-cell depletion, particularly when used in high-dose or repetitive courses, have historically been associated with increased PTLD risk. Among the newer biologic agents, high rates of PTLD presenting predominantly as primary central nervous system (CNS) lymphoma were observed in renal transplant patients who received belatacept and were EBV seronegative before transplant. Beyond these specific scenarios, it is likely that with other immunosuppressants, the risk of PTLD reflects the net state of immunosuppression in specific patients. Indeed, the duration of immunosuppression is a risk factor for late PTLD development.
Studies among pediatric SOT recipients suggest that children receiving heart transplants who are chronic high-EBV load carriers may be at significantly increased risk of late-onset EBV-positive PTLD. This level of risk might not apply to other organ recipients. , Data from prospective studies would clarify the pathogenesis and natural history of chronic viral load carriage in relationship to subsequent PTLD risk in specific allografts.
Cytomegalovirus (CMV) infection may inconsistently contribute to the net state of immunosuppression and is a known PTLD risk factor. However, more contradictory evidence exists for the role of the following as risk factors for primary disease: tacrolimus in pediatric recipients; specific human leukocyte antigen (HLA) epitopes; HLA matching; certain cytokine gene polymorphisms; preexisting chronic immune stimulation; hepatitis c infection; viral strain virulence (EBV-1 vs. EBV-2 and viral gene mutations).
Although PTLD rates increased after the calcineurin inhibitor tacrolimus became the backbone of most immunosuppressive regimens in the 1990s, it is likely that the net state of immunosuppression, an entity that is difficult to measure, is a major risk factor. Attempts to quantify the risk associated with specific immunosuppressive agents used for induction or maintenance therapy have often led to inconsistent results, which highlights the need for studies to optimize minimization of long-term immunosuppression in individual patients.
Epstein-Barr virus in hematopoietic stem cell transplantation
The epidemiology of EBV and PTLD after HSCT differs somewhat from that after SOT in that both the source of EBV and the infected B cells in PTLD after HSCT are of donor origin in the majority of cases. This is explained in part by the fact that the intense conditioning regimens used in HSCT usually eradicate host EBV latently infected B cells. The period of risk for PTLD after HSCT is also more limited compared with SOT recipients. Although EBV loads may initially become positive 3 to 4 weeks after transplant, PTLD rarely occurs in the first 30 days after HSCT, and the majority of cases occur within 6 months, with the peak incidence in the third month after transplant. ,
The incidence of PTLD after HSCT ranges from less than 1% to approximately 25% depending on the presence of risk factors. , In HSCT, unlike SOT, EBV status of the recipient is not a strong risk factor for PTLD; however, increasing donor age is a risk factor. Donor source of stem cells has been associated with risk and seems to relate to duration of lymphopenia, and hence recovery of functional T-cell immunity after transplant. PTLD is very rare after autologous bone marrow transplant. The incidence of PTLD is higher with unrelated donors than with matched related donors and increases with degree of HLA mismatching. , Because cord blood does not contain EBV-infected B cells, it was first believed that PTLD would not occur with this source of stem cells. However, the incidence of PTLD after cord blood transplant is similar to matched related donors. The time to PTLD after cord blood transplant is usually longer, likely owing to prolonged lymphocyte recovery and the need to acquire EBV from a source other than the stem cell graft. T-cell depletion (TCD) is a strong risk factor for PTLD, but it depends on the methodology of TCD. , Methods that specifically remove T cells confer a higher risk of PTLD compared with methods that deplete all lymphocyte types, including posttransplant cyclophosphamide. It is believed that in the latter approach, depletion of EBV-infected B cells in addition to T cells from the donor lowers the risk for PTLD compared with those that deplete only T cells. Development of graft-versus-host disease (GVHD) has not consistently been demonstrated to be a risk factor for PTLD, although the agents used to prevent and/or treat GVHD, such as anti–T-cell antibodies, do increase the risk of PTLD. ,
Epstein-Barr virus in cancer
Complications of chemotherapy resulting from EBV infections are very rare. However, there are anecdotal reports of EBV lymphoproliferative disease, usually associated with prolonged immunosuppressive therapy, such as maintenance therapy for acute lymphoblastic leukemia. Reducing or withholding chemotherapy allowing anti-EBV T-cell immunity to recover is often sufficient to resolve these complications. HLH can be triggered by EBV infection and can be the predominant symptom of some EBV-associated malignancies, particularly mature T/natural killer (NK) cell non-Hodgkin lymphomas (NHLs), or HLH can develop as a complication of EBV infection in patients receiving chemotherapy. In either scenario, aggressive therapy is required.
Clinical manifestations
Clinical manifestations of EBV infection range from asymptomatic illness to clinically significant and potentially life-threatening disease in children who have undergone SOT or HSCT. As noted earlier, although EBV disease is much less frequently reported in children undergoing treatment for cancer, an increased rate of primary infection compared with children without cancer has been reported to occur, and cancer therapy–associated lymphoproliferative disorders have been reported, though rarely, in the pediatric population. At least some evidence supports that EBV may be a cause of fever alone in transplant recipients and cancer patients with active EBV infection.
EBV infection can either be primary (new infection occurring in an immunologically naïve patient) or as the result of reactivation of latent EBV in the immunocompromised child. Additionally, reinfection with a new EBV strain may occur. For SOT recipients, primary infection is associated with more clinically significant disease, whereas reactivation or reinfection tends to be mild or even asymptomatic. The spectrum of clinical disease in SOT recipients includes a nonspecific febrile illness that can resemble the CMV syndrome, typical mononucleosis, and PTLD, including EBV-associated malignant lymphoma (e.g., Burkitt lymphoma [BL]). In addition, organ-specific manifestations such as enteritis and hepatitis in the absence of PTLD can also be seen. Rarely EBV has been associated with posttransplant smooth muscle tumors in SOT recipients as well, and EBV-positive T-cell PTLDs are also rare occurrences and are associated with a very poor prognosis.
The clinical presentations of the spectrum of EBV disease including PTLD frequently overlap and may affect a large number of sites within the body ( Table 18.1 ). Each of these syndromes can present with prolonged episodes of fever. Adenopathy and hepatosplenomegaly are frequently but not always seen. Gastrointestinal disease occurs frequently and is particularly common in recipients of intestinal transplantation. The presence of diarrhea associated with microscopic or gross blood warrants investigation for EBV. Hematologic changes, including leukopenia, neutropenia, and thrombocytopenia, all frequently occur. Of note, some children with EBV-associated PTLD may be asymptomatic with mass lesions involving nodes or organs found on examination or imaging. In general, variation in severity and extent of disease is believed to be related to the degree of immunosuppression and adequacy of the host immune response in the pediatric organ transplant recipient. For SOT recipients, onset of viral syndrome, mononucleosis, and polymorphic PTLD occur primarily within the first year, whereas monomorphic PTLD and lymphoma tend to occur later.
| Symptoms/Complaints | Signs |
|---|---|
| Swollen lymph glands Weight loss Fever or night sweats Sore throat Malaise and lethargy Chronic sinus congestion and discomfort Abdominal pain Anorexia, nausea, and vomiting Gastrointestinal bleeding Symptoms of bowel perforation | Lymphadenopathy Hepatosplenomegaly Subcutaneous nodules Tonsillar enlargement Tonsillar inflammation Signs of bowel perforation Mucocutaneous ulceration Mass lesions Focal neurologic signs |
In contrast to the variability and spectrum of disease in pediatric SOT recipients, the development of EBV infection before immune reconstitution places all EBV-infected pediatric HSCT recipients at risk for progression to PTLD. Clinical symptoms most frequently include fever but manifest the range of symptoms seen in SOT recipients; EBV-negative PTLD or non–B-cell disease is extraordinarily rare. Finally, there are only limited data describing the clinical presentation of EBV disease in children undergoing treatment for cancer. Although it is clear that development of lymphoproliferative disorder after primary infection is rare, data describing clinical presentation in the absence of this have not been generally reported.
Prevention of Epstein-Barr virus infection and PTLD
The impact of EBV disease including PTLD in children undergoing SOT and HSCT has prompted interest in the prevention of EBV infection and its complication in these populations. Accordingly, evidence assessing the potential efficacy and impact of preventive strategies against EBV have been published, although with few exceptions, prospective randomized comparative trials are generally lacking. Potential prevention strategies against EBV can be categorized as immunoprophylaxis, chemoprophylaxis, and preemptive therapy. In addition to these strategies, the use of leukocyte-reduced blood products also serves as an important barrier to the spread of EBV because the virus is typically carried in B lymphocytes, which are eliminated through the process of leukoreduction. Because of the relatively low frequency of recognized EBV disease in children undergoing treatment for cancer, evidence assessing the efficacy and impact of preventive strategies against EBV in this population is not available and will not be discussed.
Immunoprophylaxis
Immunoprophylaxis can be categorized as active or passive. Active immunoprophylaxis would be accomplished through use of an EBV vaccine but none is available for clinical use. Passive immunoprophylaxis can be accomplished by providing anti-EBV antibody through the infusion of intravenous immunoglobulin (IVIG) or through infusion of EBV-specific cytotoxic T lymphocytes (CTLs). Published data demonstrated a protective effect of IVIG on the development of EBV disease in a SCID mouse model. The potential role of IVIG was further supported by a large retrospective analysis that found that SOT recipients receiving anti-CMV immunoglobulins for CMV prophylaxis did not develop PTLD during the first year (during the time of prophylaxis). However, several prospective studies failed to confirm this finding. A randomized controlled trial using anti-CMV immunoglobulins prophylaxis versus placebo in pediatric liver transplant recipients did not identify significant evidence of prevention of either EBV disease or EBV-associated PTLD, although a trend toward less EBV disease and PTLD was observed in patients receiving immunoglobulins. Similarly, no impact on time free from any EBV viral load, mean viral load, time free from achieving a “high” EBV viral load, or development of EBV-associated PTLD was observed in a prospective study of IVIG versus placebo in EBV-mismatched (donor EBV-seropositive/recipient EBV-seronegative) organ recipients (adult and pediatric), all of whom received 12 months of antiviral therapy consisting of intravenous ganciclovir followed by oral antivirals (acyclovir or ganciclovir). Evidence on the protective effect of IVIG in HSCT recipients against EBV is lacking.
The use of EBV-specific CTLs as adoptive immunotherapy has been proven to be efficacious in stem cell transplant recipients, although the limited availability of access to CTL therapy affects the extent of its use. For HSCT recipients, the EBV-positive donor is often the source of the EBV-specific T cells, though third-party donors have been successfully used. Rates of prevention approaching 100% have been reported by centers with access to EBV-specific T-cell therapy. In contrast to the successful use of this approach in HSCT recipients, efforts to translate these benefits to the prevention of EBV/PTLD in SOT recipients have been limited and have not succeeded as of this time.
Chemoprophylaxis
Chemoprophylaxis using antiviral agents, such as acyclovir and ganciclovir, represents another possible approach to preventing EBV/PTLD. Unfortunately, this has not been the subject of randomized trials. Ganciclovir, or its prodrug valganciclovir, may be the preferred drug for EBV prophylaxis because of its higher in vitro antiviral activity. Nevertheless, these drugs are only effective against the lytic forms of EBV. This might translate into inefficiency in preventing latently driven disease, providing a potential explanation for the lack of clear evidence of benefit of the use of antivirals to prevent EBV. Initial support for the potential role of antiviral therapy in the prevention of EBV infection was derived from several single-center retrospective studies that, although encouraging, suffered from methodologic concerns, including lack of appropriate controls or concomitant changes in clinical practice that could have affected the development of EBV and PTLD. Although a U.S. case-controlled study suggested a potential role of ganciclovir given for CMV prophylaxis in the reduction of PTLD incidence in kidney transplant recipients, other studies have not confirmed the efficacy of ganciclovir, valganciclovir, or acyclovir against EBV/PTLD in SOT recipients. A randomized prospective trial of 2 weeks of ganciclovir compared with 2 weeks of ganciclovir followed by 50 weeks of oral acyclovir in EBV- seronegative pediatric liver transplant patients did not establish any benefit to extended use of antiviral therapy to prevent EBV disease. A 2016 meta-analysis showed that the use of antiviral drugs (ganciclovir, valganciclovir, acyclovir, and valacyclovir) in mismatched EBV transplant recipients (D + /R − ) had no effect on PTLD incidence in children and adults undergoing SOT. No significant differences were seen across all types of SOTs, age groups, or antiviral use as prophylaxis or preemptive strategy. Despite the results of the meta-analysis, definitive conclusions on the presence or absence of clinical benefits of the use of ganciclovir and related antiviral agents to prevent EBV and it complications likely require prospective multicenter randomized trials.
Because transmission of EBV from the donor to the EBV-naïve recipient is a major risk factor for EBV disease and PTLD, the role of antiviral chemoprophylaxis given to the donor to limit transmission has also been explored. Two pilot studies in the setting of adult kidney recipients of living donors provide encouraging preliminary results that treatment of the donor or recipient before transplant might affect EBV transmission and potentially PTLD. The first study was a randomized treatment of the donors with 2 weeks of valganciclovir. Although the second study used a single dose of rituximab, as opposed to an antiviral, it highlights the principle of potentially interrupting viral replication before transplantation.
Viral load monitoring and preemptive prevention strategies
Among pediatric SOT recipients, surveillance monitoring of EBV loads to inform preemptive reductions in immunosuppression has resulted in a decreased incidence of EBV/PTLD compared with historical controls. McDiarmid and colleagues reported a decreased incidence of PTLD from 10% to 5% using EBV viral load monitoring to guide the combined use of reduced immunosuppression and intravenous ganciclovir in pediatric liver transplant recipients with rising EBV loads. Other studies demonstrated decreased incidences of PTLD using decreased immunosuppression alone without ganciclovir in response to elevated EBV loads. One limitation of this approach is that the development of a detectable load does not necessarily predict progression to EBV disease, and additional risk factors that reliably add specificity to the predictive value of an elevated load beyond developing primary EBV infection after SOT have not been identified. Accordingly, reductions in immunosuppression were carried out based on elevated load alone. Owing to the risk of rejection, these reductions have been conducted carefully with ongoing attention for the presence of findings suggestive of early rejection.
Some centers have considered the preemptive use of the anti-CD20 monoclonal antibody rituximab for pediatric SOT patients with elevated EBV viral load, although little published data are available. Martin and colleagues reported encouraging results using EBV load monitoring to inform the preemptive use of rituximab in EBV D + /R − adult kidney transplant recipients. However, the majority of treated patients actually had clinical evidence of EBV disease at the time of treatment. Accordingly, these data relate more to the use of rituximab for early treatment and not prevention of EBV disease. Additional experience is needed to confirm the efficacy and long-term safety of rituximab in a prevention/preemption model against EBV in the SOT setting.
Rituximab is more often used among HSCT recipients than among SOT recipients. Because such recipients, who are at high risk for PTLD, often develop EBV infection before reestablishment of adequate cell-mediated immunity, reduction of immune suppression is frequently not an option. As the period of risk is much more predictable and relative early after HSCT, monitoring with EBV viral load polymerase chain reaction (PCR) and preemptive therapy with rituximab appears to reduce the incidence of PTLD. Although no randomized trials have been published to date, this approach of serially monitoring high-risk patients (e.g., patients who receive TCD) by weekly EBV blood PCR and giving rituximab for patients with elevated EBV DNA levels or persistently increasing levels has become standard of care at many institutions, especially for centers without access to adoptive immunotherapy against EBV (see earlier text).
In summary, it appears that the strategy of using EBV load monitoring in SOT to inform preemptive reduction in immunosuppression to prevent EBV/PTLD in SOT recipients is the optimal currently available preventive strategy, although more data evaluating the comparative safety and efficacy of rituximab with reduced immunosuppression alone in response to rising or elevated EBV loads are needed. For HSCT recipients, preemptive use of rituximab in addition to the use of prophylactic or preemptive EBV-specific T cells are current optimal preventive strategies.
Diagnosis
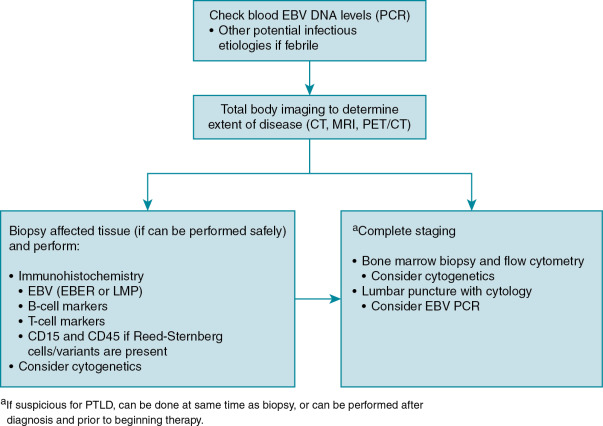
Diagnosis of EBV disease can be difficult in the setting of chemotherapy or transplantation, as it may present as anything in a spectrum of clinical manifestations from primary infection, reactivation, EBV-associated disease, or PTLD. Serologic findings can be difficult to interpret in this population with false-negative results (owing to immunosuppressive therapy), false-positive results (from receipt of blood products including immunoglobulin), or changes in titers as the result of immune dysregulation. PCR detection of virus in blood or plasma is quite sensitive but can lack specificity. Imaging, such as computed tomography scan, magnetic resonance imaging, or metabolic imaging (e.g., positron emission tomography scan), is sensitive in detection of PTLD and is useful for determining extent of disease, but specificity is poor, limiting diagnostic value. The most reliable method of diagnosis of EBV disease is tissue biopsy, and this should be done whenever it is safe to perform. When biopsy is not safe to perform, a presumed diagnosis can sometimes be made based on rising EBV viral loads and characteristic findings on imaging. An algorithm highlighting the approach to the diagnosis of EBV associated PTLD is shown in Fig. 18.1 .