Adenoviruses
In the past few decades, significant progress has been made in understanding the epidemiology of adenovirus infections and developing preventative and therapeutic strategies. However, adenovirus infections remain a clinical and diagnostic challenge. Isolation of adenovirus does not necessarily correlate with invasive disease because the virus may persist asymptomatically in lymphocytes.
Adenoviruses are classified within seven species (A through G) based on guanine and cytosine content in the DNA, ability to agglutinate red blood cells, and chemical and biophysical criteria; the species A through F circulate globally. To date, 51 serotypes and 90 genotypes have been described; genomic variants can be identified within the same serotype. About one-third of the known serotypes have been associated with disease in the immunocompetent pediatric population, with serotypes 1 through 5, 7, 21, and 41 most commonly identified. Adenoviruses display broad tissue tropism and can infect several cell types, but certain serotypes manifest as specific clinical infections ( Table 22.1 ). The predominant serotypes vary among different continents and countries and change over time as transmission of new strains replace existing dominant serotypes.
| Adenovirus Serotype | Common Disease Association in Immunocompromised Patients |
|---|---|
| 1-7; genotype 55 | Respiratory tract infections |
| 3; 7; 11; 21; 33-35 | Urinary tract infection, hemorrhagic cystitis |
| 12; 17; 31; 40; 41 | Gastroenteritis |
| 1; 3; 5; 7 | Hepatitis |
| 3-4; 7-8, 11; 14; 19; 37; genotypes 53, 54, 56 | Keratoconjunctivitis |
Species C serotypes, commonly associated with respiratory tract infections in young children, can enter a latent phase that can last years with intermittent release of live virus in stool after resolution of the primary infection. Small quantities of replicating and nonreplicating adenovirus DNA have been found in lung epithelial cells, the central nervous system, tonsils, and adenoids in the absence of acute infection; the majority of adenovirus DNA is isolated from the T-lymphocyte population. ,
Latency is characterized by evasion from immune surveillance and expression of viral proteins by the host cell without complete replication of the adenovirus. Several mechanisms contribute to latency: blockage by viral proteins of cellular apoptosis, cellular immune response, release of antiinflammatory and lytic cytokines, and downregulation of major histocompatibility complex class I molecules. Viral latency poses challenges in interpretation of detection of adenovirus DNA in stool and respiratory specimens. As humans are the only reservoir for adenovirus, the intermittent shedding of the virus in the airways and stool maintains the transmission of the viruses in the population.
Guidelines have established definitions of adenovirus infection and adenovirus disease. , Adenovirus infection is defined by detection of adenovirus in stool, blood, urine, or upper airway specimens by viral culture, antigen tests, or polymerase chain reaction (PCR) from asymptomatic patients. Adenovirus disease is defined by detection of adenovirus in biopsy specimens (by immunohistochemical stain) or from bronchoalveolar lavage and cerebrospinal fluid (by culture, antigen detection, or PCR), in the absence of an alternative diagnosis, and in the presence of attributable signs and symptoms. , In leukemia and hematopoietic stem cell transplant (HSCT) patients, adenoviral disease may be classified as probable (adenovirus infection plus corresponding symptoms and signs without histologic confirmation) or proven (adenovirus infection plus corresponding symptoms related to the infection and histologic confirmation of the virus in the appropriate location). Disseminated disease is defined by involvement of two or more organs, excluding DNAemia. , These definitions were developed mainly for consistency in outcome designation across research studies; however, the distinction between adenovirus infection and disease remains challenging in clinical practice.
Epidemiology and risk factors
Adenoviruses are isolated more frequently in pediatric than adult solid organ and hematopoietic stem cell transplant recipients, with the highest incidence in children younger than 5 years and a decreased incidence toward adolescence. , As in the general population, adenovirus infections and disease in immunocompromised hosts do not have seasonal variability, although most are diagnosed in winter and spring. , Adenoviruses can be transmitted in several ways: (1) via the respiratory route by infected aerosols, by conjunctival inoculation, by person-to-person contact, by fomites, or by the fecal-oral route ; (2) through the transplanted organ ; or (3) through reactivation of a latent infection. , The majority of infections are community acquired in immunocompetent hosts, but nosocomial transmission has been described among hospitalized pediatric recipients. , The incubation period ranges from 2 days to 2 weeks, depending on the adenovirus serotype and mechanism of transmission.
Solid organ transplantation
The incidence of adenovirus infections among solid organ transplant (SOT) recipients depends on the allograft type and degree of immunosuppression ( Table 22.2 ). The majority of the infections are diagnosed within the first few months after transplantation, , , but late infections have been described in pediatric SOT recipients. , Overall, infections are diagnosed early after transplantation at a median time of 1 month (0.5 to 10 months) in one study and 1.64 months (0.03 to 153 months) in another study. Early infections can suggest donor-derived infections, viral reactivation, or nosocomial infection.
| Allograft Type | Reported Adenovirus Incidence (%) |
|---|---|
| Kidney | 11 |
| Liver | 3.5-38 |
| Heart/lung/heart-lung | 7-50 |
| Intestinal, multivisceral | 4.3-57.1 |
Risk factors for adenovirus disease are not well established in solid organ transplantation. Adenovirus serologic mismatch seems to be a potential risk factor, but standard screening of adenovirus serology and risk stratification based on donor and recipient serostatus are not currently indicated. Younger age seems to be an independent risk factor, especially children younger than 5 years, most likely because they are immunologically naïve and have higher exposure rate. , , Pinchoff and colleagues reported adenovirus disease only in the pediatric intestinal transplant recipients in their cohort, whereas invasive disease did not develop in any of their adult intestinal transplant recipients during the same study period. Adenoviruses persist in tonsillar lymphocytes in about 80% of children investigated, and the number of adenoviral genomes per lymphoid cell tends to decline with age. Allograft type has an indirect correlation with the risk of adenovirus infections. Allografts with large amounts of lymphoid tissue (such as the intestine) pose a high risk of rejection, requiring more intense immunosuppressive regimens. , This lymphoid tissue could also be a reservoir of adenovirus. The degree of immunosuppression seems to be directly correlated with the rates of adenovirus infections; the highest prevalence of infections is reported early after transplantation. , Immunosuppression affecting more T-cell–mediated immunity, such as lytic therapy (OKT3 or ATG) for induction or steroid-resistant rejection , , seems to be play an important role. The infections tend to resolve with reduction in immunosuppression. A lower absolute lymphocyte count might be a risk factor for adenovirus disease but not adenovirus infection.
Few risk factors for progression from asymptomatic infection to adenovirus disease have been described in solid organ transplant recipients:
- 1.
Detection of the virus in the first months after transplantation
- 2.
Repeated detection of adenovirus from the same site
- 3.
Identification of adenovirus from two or more sites
- 4.
Initial high adenovirus DNAemia, although a clear threshold has not been established
- 5.
Intensification of immunosuppression, and
- 6.
A more than 10-fold rise of viral load in the blood might be associated with fatal disease. , ,
Based on the available data, the latest American Society of Transplantation guidelines do not recommend routine screening for adenovirus DNAemia as it is unclear if asymptomatic DNAemia would require treatment, and the side effects associated with cidofovir use would outweigh the benefits.
Hematopoietic stem cell transplantation
Adenovirus infection rates between 8% and 28% have been reported in pediatric recipients of a HSCT, considerably higher than in adults (3% to 15%). Fifty percent of these infections progress to disease with a case-fatality rate exceeding 50%. Adenovirus in autologous HSCT patients is relatively rare, whereas infection is more common in allogeneic HSCT patients, particularly in the first 100 days after transplant. In 12 screening studies performed in children undergoing allogeneic HSCT, the incidence of adenovirus DNAemia ranged from 6% to 28%. Younger age is associated with an increased risk of adenovirus infection and disease. , , One retrospective study of 328 pediatric allogeneic HSCT patients found that children younger than 5 years 2.3 times as likely to develop adenovirus infection than those older than 5 years.
Risk factors for adenovirus infection and disease are well established in pediatric allogeneic HSCT. They include the following: (1) use of T-cell depletion, (2) unrelated donor graft, (3) unrelated cord blood graft, (4) grades III and IV graft-versus-host disease (GVHD), and (5) severe lymphopenia (<300 CD3 cells/μL of peripheral blood). , , Treatment with the anti-CD52 antibody alemtuzumab or antithymocyte globulin is also described as an independent risk factor of adenovirus infection. Lack of adenovirus-specific T cells, and more generally, delayed recovery of lymphocyte count, is associated with delayed clearance of adenovirus infection.
In pediatric allogeneic HSCT recipients, onset of invasive adenovirus infection and disseminated disease is often preceded by replication and detection of the virus in the gastrointestinal tract. , In one study of 138 pediatric recipients of a HSCT, rapidly increasing viral copies in serial stool specimens, particularly exceeding log 6.0, preceded onset of DNAemia by a median of 11 days. Additional studies have confirmed the phenomenon of stool viral replication preceding DNAemia, although the threshold for stool copy number varies based on the molecular method used. Another study correlated stool adenovirus copy number with histopathology for biopsy samples in pediatric HSCT patients and found that persistent adenovirus infection in gastrointestinal lymphoid tissue, particularly in the terminal ileum, correlated with adenovirus recurrence after transplant. Critically high viral loads in stool appeared within the first 3 weeks after HSCT. These data suggest that persistent adenovirus infection in the intestine represents an important risk factor for disease after transplant and serve as the basis for screening and preemptive treatment in high-risk pediatric HSCT recipients.
Clinical manifestations
In immunocompromised patients, adenovirus can be asymptomatic or cause varying disease manifestations, including conjunctivitis, acute respiratory illness, gastroenteritis, urinary tract infections, or disseminated disease. These manifestations tend to be more severe in pediatric transplant populations, including respiratory failure as the result of pneumonitis, hemorrhagic cystitis, neurologic disease, and multiorgan failure.
Solid organ transplantation
Similar to HSCT recipients, detection of adenovirus in SOT recipients can be without symptoms or be associated with local and disseminated disease. The risk for adenovirus disease is variable by type of allograft. Published data suggest that detection of adenovirus in the blood in liver and intestine transplant recipients might be associated with increased risk of developing sepsis. In a study by de Merzeville and colleagues, the most frequently involved site of disease was the gastrointestinal tract (75%), followed by respiratory tract (21%), blood (21%), and liver (7%); the most common clinical manifestations are diarrhea (68%) and fever (53%).
In most SOT recipients, the allograft is the most likely site of disease. , It is possible that the allograft is more vulnerable, owing to the local immunologic environment (low-grade, subclinical GVHD and rejection) or reactivation of the virus from the donor or recipient lymphoid tissue. In some studies, adenovirus detection has been linked with subsequent acute rejection, possibly caused by activation of the cytokine release by stimulating the cellular immune response and changes in immunosuppressive regimens. , However, not all studies support this association. , ,
In liver transplant recipients, infections tend to be diagnosed early, with severe disease seen predominantly in the first 2 months, , but late infections have been reported. The median time to adenovirus infection after transplantation ranges from 25.5 days to 61 days. , Frequent symptoms on presentation noted in previous studies were fever, rhinorrhea, diarrhea, and blood in the stool. , , Hepatitis was the most common presentation, with liver enzymes aspartate transaminase and alanine transaminase) peaking in thousands with aspartate transaminase higher than alanine transaminase. , , However, patients can also present with stomatitis, rash, enteritis and/or colitis, hemorrhagic cystitis, nephritis, and pneumonitis and can progress to sepsis. , , , Pneumonitis is less common but can progress to adult respiratory distress syndrome and is associated with high mortality. , The case-fatality rate in patients with adenovirus hepatitis is high, approximately 63% reported in a small case series, including mainly pediatric patients; mild elevation of the liver enzymes and limited necrosis on the initial biopsy correlate with better survival.
Intestinal transplant recipients present with fever, rhinorrhea, blood in the stool, and increase ostomy output; a significant proportion of these patients progress to disseminated adenovirus disease. , , , Not all patients in whom invasive disease develops have prior asymptomatic infection. Median time from transplantation to adenovirus disease varies in different studies from 24 days to 113 days. , Adenovirus disease tends to be diagnosed more frequently in the first 6 months after transplantation at similar rates in isolated intestinal transplant and multivisceral transplant recipients. Enteritis is a common presentation, and it can be challenging to distinguish it from acute cellular rejection, especially because adenovirus enteritis is often preceded by treatment for rejection. In patients with increased stool output and blood in the stool, an endoscopy with biopsy to evaluate for rejection and stool testing to evaluate for gastrointestinal infections (including adenovirus) are indicated. The ileum is the most common site of infection, but jejunum and colon can also be involved. Adenovirus ascending cholangitis seems to be a rare complication of the gastrointestinal infection. Morbidity and mortality can be attributed to adenovirus disease and to bacteremia and sepsis owing to compromise of intestinal epithelium integrity. Case-fatality rates have been reported as high as 45%.
In lung transplant recipients, adenovirus can cause acute flu-like illness but patients often present with allograft infection, diffuse alveolar damage, or necrotizing pneumonia. In addition, adenoviruses have been associated with chronic allograft dysfunction owing to bronchiolitis obliterans, interstitial fibrosis or bronchiectasis, need for retransplantation, and death. However, these associations have not been confirmed in large comparative studies.
In heart transplant recipients, detection of adenovirus genome in endomyocardial biopsy specimens was associated with adverse cardiac events in the short term, and it was an independent predictor of intermediate to long-term allograft dysfunction or loss. Adenovirus has been associated with posttransplantation coronary vasculopathy, but the mechanism for this association has not been delineated. Based on the concern that persistence of adenovirus in the endomyocardium is responsible for subclinical inflammatory response, some have suggested that maintenance of steroid therapy and the use of intravenous immunoglobulin might reduce the infiltration and the complications; however, there are limited data to support this approach.
Adenovirus infections are infrequent in kidney transplant recipients and usually present with hematuria, dysuria, fever, or respiratory symptoms. Adenovirus DNAemia was reported in 14.7% of pediatric kidney transplant recipients at a median of 173 days (interquartile range [IQR] 109 to 310 days); the median duration of DNAemia was relatively long 55 days (IQR 36 to 79 days). Adenovirus disease in renal recipients seems to present later (at a median of 309 days after transplantation, IQR 258 to 360 days) and is associated with a longer duration of DNAemia (median of 79 days, IQR 55 to 97 days). Hemorrhagic cystitis is the most common manifestation in pediatric populations and is generally a self-limited illness. Graft dysfunction is uncommon and should be differentiated from BK virus nephropathy and rejection. Orchitis, gastroenteritis, and pneumonia have been described in renal transplant recipients.
Hematopoietic stem cell transplantation
In HSCT recipients, clinical manifestations vary based on the risk factors present, including age, type of graft, level of immunosuppression, and timing after transplant. Symptoms of adenovirus disease most often manifest within the first 100 days after transplant. , Fever and diarrhea are the most common symptoms reported, followed by elevated liver enzyme levels and secondary pancytopenia. Gastrointestinal symptoms range from mild diarrhea to hemorrhagic colitis , ; diarrhea can mimic gastrointestinal GVHD, causing diagnostic uncertainty in allogeneic transplant patients. Respiratory symptoms can vary from mild, nonspecific cold-like symptoms of the upper tract to severe pneumonia. , Urothelial involvement usually manifests as hemorrhagic cystitis, which rarely progresses to disseminated infection. Additional reported symptoms include nephritis, hepatitis, encephalitis, myocarditis, pancreatitis, and multiorgan involvement, the latter of which is frequently associated with hepatic failure. Fatal adenovirus disease is reported in 13 to 50% of infected patients.
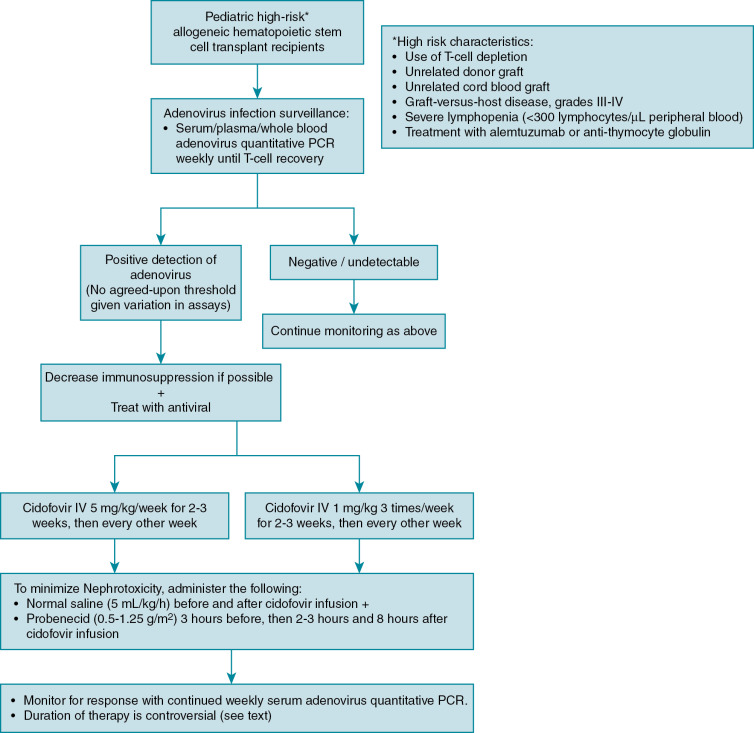
Disease prophylaxis/prevention
Because of the high mortality from localized and disseminated disease in high-risk allogeneic HSCT patients, guidelines exist to direct screening and preemptive therapy for adenovirus in high-risk groups. , , Monitoring in autologous and standard-risk allogeneic HSCT patients, such as those receiving human leukocyte antigen–identical sibling transplants, is not routinely recommended. Defining which allogeneic HSCT patients are at high risk varies slightly among guidelines, but generally includes pediatric recipients of T-cell–depleted grafts, unrelated donor grafts, cord blood grafts, severe GVHD, severe lymphopenia, and treatment with alemtuzumab and antithymocyte globulin ( Fig. 22.1 ). , , An algorithm for adenovirus surveillance and treatment is provided (Fig. 22.2). As adenovirus screening of stool specimens is not widely available, weekly monitoring of serum adenovirus PCR immediately after transplant is recommended until immune reconstitution ( Fig. 22.1 ). , Preemptive treatment when DNAemia is detected is discussed in the treatment section.