Mycobacterium tuberculosis infection
Epidemiology, risk factors, and pathogenesis
Mycobacterium tuberculosis ( MTB) is among the most successful respiratory pathogens worldwide as the majority of those infected are asymptomatic and will live their lifetime without clinical manifestations. Latent infection is defined as having asymptomatic MTB infection, as the immune system can control but not eradicate the pathogen. In low-incidence areas, latent infection is treated to reduce the risk of reactivation occurring later in life. Those with active tuberculosis (TB) disease (either as primary infection or reactivation) are typically symptomatic or have microbiologic (including sputum or other sterile site) or radiographic evidence of disease. Immunosuppressed hosts can have minimal symptoms or symptoms that are difficult to discern from their underlying disease, so a heightened level of clinical suspicion and knowledge of disease in these high-risk patients are necessary.
The risk of TB among adult solid organ transplant (SOT) recipients is approximately 4 to 74 times greater than the general population. , Risk should always be referenced with local TB incidence as rates can vary substantially by geographic location. For example, TB prevalence rates among SOT patients are reported between 0.4% and 6.6% in populations with low TB risk (e.g., United States and Western Europe) compared with rates as high as 15.2% in areas with high TB rates. The TB-associated mortality among SOT recipients ranges from 6% to 29% , compared with the less than 5% mortality attributed to TB in the United States. Mortality rates are higher in renal transplant recipients and especially those with graft rejection, miliary TB, and those receiving T-cell–depleting immune suppression. Although there are only limited data, the estimated mortality in pediatric SOT TB cases was more than 30% in a small series.
Risk factors associated with TB among SOT recipients are multifactorial. Lung transplant recipients appear to have the highest incidence of TB among organ transplant types, followed by liver and then kidney transplant recipients. Other risks include a history of prior TB, the use of T-cell–depleting antibody as well as immune modulators that impair T-cell function (e.g., basiliximab, alemtuzumab), chronic renal insufficiency, graft rejection, augmented immune suppression, diabetes mellitus, hepatitis C, chronic liver disease, increased recipient age, and presence of other co-infections (e.g., cytomegalovirus, Pneumocystis jiroveci pneumonia). , , , Among renal transplant recipients, the incidence of TB was associated mammalian target of rapamycin (mTOR) inhibitors or azathioprine use. Higher rates of TB are observed in those who are older at the time of transplant, as older patients are more likely to have latent infection due to having lived in an era where TB was more prevalent. , Recognized behavioral risk factors include increased cumulative time residing in high–TB-endemic countries and exposure within prisons or homeless populations. Independent risk factors of mortality among SOT recipients with TB include the presence of acute cellular rejection after the diagnosis of MTB infection, use of three or more TB drugs (likely reflecting active TB), and disseminated MTB.
Four different mechanism of pathogenesis exist in SOT recipients:
- 1.
Reactivation TB, in which the recipient has preexisting latent infection before SOT is believed to be the most common etiology in non–TB-endemic areas like the United States. This is more common in adults compared with children, as adults are more likely to have latent MTB.
- 2.
Donor-derived transmission either from a donor who had unrecognized active TB or latent infection. The reported risk of TB transmission is estimated at 30% when a donor has active TB. Although lung transplants are recognized to have the greatest risk of donor-derived TB transmission, given that the lung is the most common organ to harbor MTB, all SOT types have been associated with donor MTB transmission. In the United States, donor-derived TB accounts for less than 5% of TB cases after transplant , and estimates are likely higher in countries with more TB-endemic rates.
- 3.
Active TB in a recipient who is either unrecognized or recognized (undergoing treatment but requires emergent transplant).
- 4.
Primary infection that occurs after the time of transplantation.
The overall contribution of each listed category of TB transmission is not well characterized and it remains unclear what the actual risk of TB disease is associated with each potential transmission modality. Thus the overall TB risk is likely cumulative based on the contribution of both the recipient and donor risk factors as well as the endemic TB rates inherent to the geographic area, behavioral risk, and degree of immune suppression.
Very few data are available for pediatric SOT populations as significantly fewer children undergo transplant compared with adults. In general, the risk of developing active TB is greatest among those younger than 5 years and then during adolescence/early adult ages. The incidence of TB after pediatric SOT has been reported as high as 2.4% in nonendemic areas to 9.7% in high–TB-endemic areas. Within the same geographic area, the rates among adult SOT recipients are higher than in children after SOT and are likely due to the fact that transmission in children is more likely from primary infection (recent exposure) and less likely from reactivation and other transmission modes discussed previously.
Hematopoietic stem cell transplant (HSCT) is an independent risk factor for TB, and it is estimated that HSCT recipients have a 10 to 40 times greater risk than the general population. , The incidence of TB varies based on geographic area with rates estimated at 0.1% to 5.5% in low-endemic areas but 16% in high endemic areas. , For example, in India, a high endemic area, nearly 2% of all infections after HSCT are due to TB. Risk factors include allogeneic transplantation with unrelated donors or mismatched donor, graft-versus-host-disease (GVHD), use of total body irradiation, busulfan, cyclophosphamide, corticosteroid treatment, T-cell–targeted immune modulators, or prior history of TB. , Although there are no direct comparisons, it is generally accepted that HSCT recipients are at less risk of TB than SOT recipients. Despite HSCT recipients being more severely immune suppressed before and during HSCT compared with SOT recipients, the duration of immune suppression is only transient until engraftment ensues. This contrasts with SOT recipients, who require more prolonged (in many cases a lifetime of) immune suppression to prevent graft rejection. Moreover, donor-derived transmission of MTB is rare; the primary mode of pathogenesis is either reactivation from underlying latent infection or primary infection immediately before or during HSCT. Mortality from TB ranges from 0 to 50%. Like adults, children who have undergone HSCT are believed to be at higher risk of TB than the general population, although data are not well defined. While data are limited in pediatrics, some have reported that the risk of TB is lower in HSCT than SOT recipients. Up to 25% of pediatric TB patients do not have an obvious risk factors for TB, and there are no universal screening practices in pediatric HSCT candidates as most hematology/oncology physicians believe that the tuberculin skin tests (TSTs)/interferon-gamma release assays (IGRAs) do not work in HSCT candidates receiving immune suppression. More efforts are needed to better characterize TB in both adult and pediatric HSCT recipients.
Clinical manifestations
Variable presentations and delays in diagnosis likely contribute to the higher TB mortality rates observed in SOT. Most cases of posttransplant TB occurred within the first 6 months of transplant (reported medians of 4 to 9 months) with the exception of renal transplant recipients, in whom TB occurred later. , , The interval of time between SOT to clinical presentation of TB appears to be associated with transmission modality as earlier onset of disease is associated with reactivation or donor derived, whereas later onset of disease likely occurs from primary infection. In pediatric SOT cases of TB, the reported time intervals between transplant and diagnosis was 8 months to 8 years and were presumed to be predominantly from primary infection after transplant. , Presenting symptoms of TB may be nonspecific and associated with site of involvement (∼24% of cases). Although pulmonary TB is the dominant manifestation in the general population, extrapulmonary disease is more prominent in SOT patients (79% to 84%). Miliary disease was reported in 44% to 63% of SOT TB cases, pulmonary disease in 12.2% to 36%, and genitourinary disease in 4% of cases. , Other reports showed extrapulmonary disease or disseminated (35% to 67%) as the most common manifestation of TB in SOT recipients and included involvement in the liver, gastrointestinal tract, lymph nodes, pericardium, bone, orbital, central nervous system, and genitourinary sites. , , , Fever was reported in 64% of cases with localized disease and 94% of disseminated cases in addition to weight loss and night sweats. Many have suggested that TB should be considered in any SOT recipient with prolonged fever, especially in the first 3 months after transplant. Renal transplant recipients often present with increased creatinine as the first sign of MTB, which can progress to disseminated disease. Some have suggested that donor-derived disease more commonly presents as sepsis and organ dysfunction when a nonpulmonary organ is involved. Thus a high index of clinical suspicion is important as most patients may have atypical clinical presentations. Clinical manifestations in children were also highly variable as more than 50% of reported cases had extrapulmonary disease. In a series of seven cases of TB among children after renal transplant, almost all presented with fever, cough, and most had weight loss. In another series, four of six children presented with fever of unknown origin (with cervical lymphadenopathy or deafness/meningitis).
Unlike SOT recipients, clinical manifestations of TB in HSCT recipients are primarily pulmonary (87%), including a spectrum of symptoms that can be indolent or overt with fever, cough, dyspnea, and hypoxia. Similar to the workup for SOT recipients, a wide range of symptoms can occur with TB and a high clinical suspicion is necessary for evaluation and workup. The median time to diagnosis from HSCT was 1.22 years (range between 22 days and 4.63 years).
Diagnosis
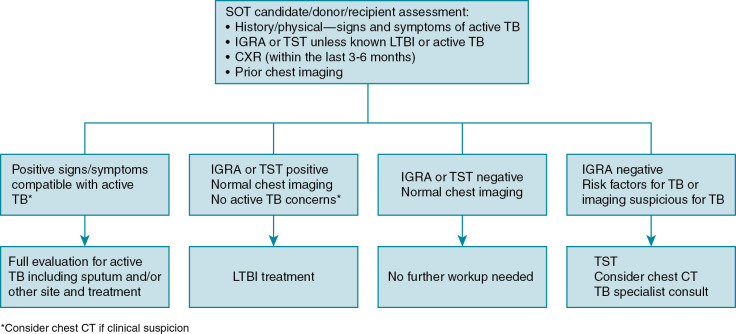
Currently, there are no universal screening/evaluation guidelines for TB among SOT candidates and recipients, although most organizational bodies include a thorough history (including an individualized risk assessment for TB), physical examination, and IGRA or TST at a minimum. , Some experts also recommend a chest radiograph within 3 to 6 months of transplant and review of any prior chest imaging to be more thorough ( Fig. 16.1 ). The experience from TB-endemic areas has suggested that the most accurate screening for active TB should not only include not IGRA/TST, sputum, careful evaluation of patient symptoms, and history but also the patient’s prior TB contact history and chest imaging. Chest radiography within 3 to 6 months before transplant is recommended regardless of TST/IGRA results in such cases. Recent exposure to a TB contact and chest radiographic evidence of prior TB were both factors that increased the risk of posttransplant TB. Pretransplant chest computed tomography (CT) evidence of “healed” or prior TB was more predictive of posttransplant TB than chest radiographs (which in some cases were interpreted as normal). For this reason, chest CT should be considered in patients for whom there is a high clinical suspicion despite negative test results. That said, imaging modalities are not diagnostic and must be considered in the context of other TB-specific testing. Above all, it is important to understand that the current existing assays for MTB are limited and no assay has 100% negative predictive value. When appropriate, a high level of suspicion is required during the evaluation.
TSTs and IGRAs are currently the mainstay of diagnostic assays but are only able to detect MTB infection without distinguishing active TB or latent infection. TST is inexpensive and there has been long-standing experience with this test for decades in all age groups; however, it has many disadvantages. The TST requires a return visit at 48 to 72 hours to measure induration, which can be subjective and false-positives can occur as a result of cross-reactivity with non-tuberculous Mycobacterium and BCG bacilli Calmette-Guérin (BCG) vaccine. The IGRA (either in the form of QuantiFERON (QFT [Qiagen]) or T-Spot.TB (Oxford Immunotec) measures the production of interferon-gamma in response to MTB-specific antigens and therefore has less cross-reactivity with nontuberculous Mycobacterium and BCG vaccination. These assays require only a single visit with an objective result (i.e., positive, negative, or indeterminant) with improved specificity over the TST. However, the IGRA is more expensive and requires a laboratory infrastructure to perform the assay. Both the IGRA and TST assays are dependent on immune-specific responses against MTB and are therefore not optimal in patients with underlying immune suppression, the population at most risk for TB disease. Even among immunocompetent hosts, these assays have limited diagnostic yield, in each, in part, owing to the lack of gold standard and the inherent testing bias in each population tested. In general, the pooled sensitivity of TST, QFT, and T-Spot.TB assays is estimated at 77%, 84% and 88%, respectively. The pooled specificity of TST, QFT and T-Spot.TB assays is estimated at 97%, 98%, and 93%, respectively, although reports vary based on risk of TB in study cohort, state of infection studied, and so on. The sensitivity and specificity of these tests have not been thoroughly tested in SOT candidates and recipients. Patients with end-stage liver disease are known to have false-negative TST results owing to cutaneous anergy and high rates of indeterminant results by IGRA. Concordance between IGRA and TST assays was compared among 1500 SOT adult recipients; fewer positive TST results were observed among SOT recipients compared with IGRA results but this did not reach statistical significance. Although limited, studies thus far have shown that a negative IGRA result before SOT was associated with a low risk of active TB after SOT. Thus many experts have preferred IGRAs over the TST as they may be more sensitive (especially in cases of end-stage liver or renal disease). Even less is understood regarding the interpretation and predictive value of the IGRAs and TST in HSCT recipients as there are little published data. One report suggested that the IGRA may be more sensitive than the TST with a high degree of discordance found between the two assays. Regardless, a negative IGRA result does not rule out the diagnosis of active TB as numerous case reports have described patients with culture-proven TB despite a negative IGRA test result. Unfortunately, more recent host biomarker diagnostics in TB, focused on biomarkers/host-dependent signatures (i.e., host gene expression profiles from blood, serum proteins, or metabolites), are still in development but are likely to be prioritized for healthy adults or those with human immunodeficiency virus (HIV) and not targeted for SOT or HSCT recipients.
Among immunocompetent children with active TB, the pooled sensitivity of the TST, QFT, and T-SPOT.TB tests was estimated to be 80%, 83%, and 84%, respectively. Similarly, the pooled specificity of the TST, QFT, and T-SPOT.TB tests were from 95%, 91%, and 94%, respectively. Some smaller studies have shown that a positive IGRA result was a more accurate predictor of active TB after recent infection. In a report of 30 pediatric SOT candidates with end-stage renal disease (all of whom were vaccinated with BCG at birth, median age 8 years), results of TST and IGRA testing were concordant in all but 1 case. The TST result was positive in all six pediatric TB SOT cases in a small series; IGRA was performed in only two and both had indeterminant results. Some experts suggest that both IGRA and TST be performed in children with high-risk families, and a positive test result from either assay should warrant further evaluation. It is worth mentioning that these reports were conducted when IGRAs were approved for children 5 years and older. The assay was recently approved for children younger than 2 years and a newer version of the QFT (i.e., QFT-plus) is being marketed. As assays continue to advance and upgrade, the sensitivity, specificity, and hopefully, predictive value will also improve. Regardless, more data are needed to assess the value of TST and IGRA in the pediatric SOT and HSCT populations, as there are currently no compelling data to suggest that one assay is better than the other.
Because of the prominence of both pulmonary and extrapulmonary manifestations in SOT patients, diagnostic imaging is critical as well as the consideration of the type of organ transplanted. In one report of TB among liver transplant recipients, the chest radiographic pattern was identified as the most important risk factor for TB. In another review of SOT-associated TB cases, all pulmonary cases were observed in lung transplant recipients, in whom as many as 75% of all cases reported had some radiographic abnormality. Chest radiographic findings in TB SOT cases can vary widely, including focal infiltrate, miliary pattern, nodules, cavitary disease, pleural effusion, or diffuse interstitial infiltrate. , CT is a more sensitive measure of disease. Patterns reported in SOT-associated TB cases include pulmonary findings of ground-glass opacity, consolidation, cavitation, tree-in-bud pattern, mediastinal lymph node enlargement, and miliary pattern. Extrapulmonary disease may have variable yield as only 30% of renal transplant recipients with TB had pulmonary findings and only 30% had lymph node enlargement.
HSCT recipients with active TB are most likely to have pulmonary manifestations. Chest radiographic patterns are similar to those as the general population, such as airspace consolidation or nodules, although unusual patterns such as diffuse alveolar hemorrhage has been reported. Chest CT findings such as consolidation, nodules, tree-in-bud, ground-glass appearance, cavitary formation, and lymphadenopathy have been described and are common. Although pulmonary involvement is common, some authors have reported higher rates of extrapulmonary disease (as high as 46%) compared with the general population.
A high level of clinical suspicion and low threshold for microbiologic testing/confirmation (either by sputum or biopsy) should be maintained when evaluating for TB in a SOT recipient. Acid-fast smear and culture (with susceptibility testing) from sputum or sterile sites suspected for MTB involvement are considered the standard microbiologic methods. The limited sensitivity and slow growth of MTB by standard culture methods suggest that early investigation and empiric treatment may be required for optimal management. To date, standard clinical laboratory–based nucleic acid amplification methods are reliable only when performed on acid-fast bacillus smear–positive samples for which a high bacterial burden is present. The advent of the GeneXpert assay (Cepheid Inc.) (and now new version, Xpert MTB/RIF Ultra) as a rapid method of MTB detection (cartridge-based, nucleic acid amplification) and isoniazid (INH)- or rifampin (RIF)-resistance testing on sputum samples has markedly improved TB diagnosis and treatment strategies worldwide. This assay has been endorsed in by the World Health Organization (WHO) for use in HIV-positive patients, and testing is currently being expanded to other biologic specimens (e.g., cerebrospinal fluid). These assays have evolved as the primary diagnostic method in areas where standard acid-fast bacillus smear and culture testing are not available. The yield in pediatric samples (pooled sensitivity of only 66%) is less optimal given the paucibacillary nature of TB disease in children compared with adults, and studies exploring the use of the GeneXpert test in other samples, such as bronchoalveolar lavage samples, pleural fluid, and other areas, have not been encouraging. Other non–culture-based assays are under development. For example, the Alere Determined TB LAM Ag assay (Abbott) is a non–culture-based assay that detects urinary lipoarabinomannan, an antigen of MTB, and has been endorsed by the WHO for HIV-infected patients with CD4 T-cells counts below 100 cells/μL in whom pulmonary or extrapulmonary TB is suspected as the bacterial burden and degree of dissemination is presumed to be high. The utility of these newer diagnostic assays has not been assessed in SOT or HSCT recipients to date.
Treatment
Active pulmonary TB should be treated with the standard regimen of INH, RIF, ethambutol (ETH), and pyrazinamide (PZA) for 2 months followed by 4 months of INH/RIF for drug-susceptible disease (with pyridoxine). Prolonged treatment may be necessary if clinical or microbiologic response is suboptimal or if central nervous system involvement or other circumstances occur. Unfortunately, this regimen (specifically the rifamycins) interacts with many of the immunsuppressive drugs used to prevent graft rejection, including calcineurin inhibitors (cyclosporine, tacrolimus), mTOR inhibitors (sirolimus, everolimus), and steroids. The inadvertent reduction in immunosuppressive drugs increases the risk of rejection, a recognized event during TB treatment among SOT recipients, although other reports have cited similar rates of rejection regardless of RIF use. Direct liver toxicity from INH, RIF, and/or PZA can occur and close monitoring of liver function test results is recommended. SOT recipients are at further risk of drug-induced toxicity; ETH, PZA, and INH require renal clearance and renal insufficiency is a common comorbidity in these patients. , In a meta-analysis review, 30 of 88 liver transplant recipients with TB had to stop or change medications because of adverse drug effects. The mean time to occurrence was 3.1 months from initiation of TB treatment. The majority of those cases (73%) were due to hepatotoxicity, whereas the remaining were due to drug interactions with immunosuppression. Those with rejection were more likely to have hepatoxicity. To prevent the risk of rejection, close monitoring of immunosuppressant drugs (e.g., cyclosporine, tacrolimus) is required as well as empirically increasing steroid levels by 50% until a period of stability is observed. RIF induction of cytochrome P450 reduces the drug levels as early as a few hours after RIF administration with maximal effect in 1 to 2 weeks. It is not unusual for the drugs such as cyclosporine or tacrolimus or mTOR inhibitors to increase two- to fivefold while RIF is used. The effects of RIF slowly decline over 2 weeks after it has been stopped. Similar issues arise among HSCT recipients who require treatment for active or latent infection as RIF will decrease levels of immunosuppressant agents and can lead to worsening GVHD. It is not uncommon to taper the cyclosporine and add corticosteroids during this period among HSCT recipients being treated for TB. Despite rifabutin having less cytochrome P450 induction, immunosuppressant medications can still be difficult to maintain. It is important to keep in mind that RIF, INH, and PZA are critical backbones in the shortened TB regimen of 6 months. Although the RIF-sparing regimens may seem more attractive, they are less effective, requiring longer treatment durations (e.g., 12 months). Similarly, PZA can be replaced with a quinolone but requires longer treatment durations. Thus standard TB treatment regimens should be encouraged as much as possible in SOT recipients with constant vigilance toward drug interactions and toxicity. Lastly, some experts have suggested that IGRA conversion results from positive to negative could be used as a surrogate for monitoring treatment response. However, although most studies show a reduction in the IGRA response to MTB antigens during treatment, the responses were variable and therefore not useful for drug treatment monitoring, especially in immunocompromised SOT recipients.
There are a number of different first-line treatment regimens for latent MTB infection that include 9 months of INH, 4 months of RIF, and 12 weeks of INH and rifapentine. In a randomized trial among adults, 4 months of RIF was associated with lower rates of hepatotoxicity compared with 9 months of INH (0 vs 8%). Alternative regimens include 6 months of INH, 4 months of rifabutin, or 3 to 4 months of INH/RIF but these regimens have not been well studied. Pyridoxine should be given in any regimen when INH is used. The use of levofloxacin with ETH was compared to INH in SOT recipients with latent infection but the trial was stopped early because of high rates of adverse events in the levofloxacin group. Although INH has higher rates of hepatoxicity, fewer drug interactions may make it more attractive than RIF despite the longer treatment duration. Timing of when to start treatment may also depend on the stability of the patient’s condition, liver function, and immunosuppressed status as drug interactions will likely cause some fluctuation. Unlike the treatment of active TB, for which treatment should begin immediately, many experts have deferred treatment of latent infection. Decisions on treatment regimens must be individualized based on baseline liver function, potential drug-related interactions, toxicity risk, compliance, and so on. Some have recommended monitoring liver function testing with prothrombin time every 2 to 4 weeks during INH treatment and then monthly once laboratory findings and clinical status are stable. Treatment with RIF should include monitoring of a complete blood count.
The treatment regimens are the same in children with the same concerns regarding drug interactions and toxicity. Although the incidence of drug-induced hepatotoxicity is generally less common in healthy children compared with healthy adults, the incidence in pediatric SOT patients is not well described and monitoring as described earlier would be reasonable. In a report of six cases of pediatric SOT TB, half used RIF treatment (all had INH and PZA in their treatment regimen). Hyperuricemia developed in two patients related to PZA; transaminases increased 2 to 7 times higher than baseline presumably from INH in four of six cases. More data are needed to assess the true risk of TB treatment in pediatric SOT recipients.
Prevention and screening practices
Screening practices among SOT candidates and donors can be difficult and vary based on epidemiologic risk and duration of exposure time in both the donor and recipient. For example, donors may be divided into low, moderate, and high risk for TB based on social history (e.g., homelessness, prison, alcohol use, low body mass index, known TB contact) and prior exposure or residence in other countries. The risk of TB can vary based on the type of SOT, choice of immunosuppression, and genetic predisposition of the recipient. Thus the guidelines discussed later vary based on endemic epidemiologic risks, experience, and practices of each institution within a given region.
As discussed earlier, screening of SOT candidates plays a key role in preventing reactivation TB after organ transplant ( Fig. 16.1 ). In a meta-analysis of liver transplant–associated TB, 35% of TB cases a prior positive TST result but were not treated. All candidates should be screened either with TST or IGRA (especially those with prior BCG vaccination), risk assessment of possible exposure (e.g., homeless, recent exposure to active TB), and chest raidography. Some experts have suggested the use of IGRAs only in low-incidence areas to prevent false-positive results, whereas others have argued that both tests should be used to maximize the sensitivity of both screening modalities. Similar to the evaluation of SOT candidates and recipients, some have suggested both TST and IGRA can be used if there is high pretest probability for latent infection and any positive IGRA or TST result should be further evaluated ( Fig. 16.1 ). Chest CT has been used to detect MTB-suspicious lesions in the lung, especially in high TB-endemic areas. The Tuberculosis Network European Trials (BNET) Consensus Committee (largely European experts) developed broad-based guidelines based on local endemic TB rates that include empiric treatment for latent infection without screening in areas with high TB-endemic rates (>100 per 100,000). As for HSCT candidate screening, both the IGRA and TST have reduced sensitivity in the setting of a person undergoing evaluation for HSCT who is already receiving chemotherapy.
Although the experience and data are limited, the guidelines for pediatric SOT candidates are essentially the same as the earlier adult guidelines with some caveats. As IGRA assays are not approved for children younger than 2 years, TST remains the primary diagnostic in very young children. Results of the TST should be interpreted regardless of BCG vaccination as it is not possible to disprove the positive test results. Unlike adults, whose prior experience and travel contribute to the risk of MTB exposure specific to the patient’s risk, a thorough assessment of risk factors among the parents and other household contacts is important as they are most likely to transmit MTB to the child. Close contacts or family members were the source of MTB infection in 66% to 80% of pediatric TB or latent SOT cases. , Similarly, children whose parents were born outside the United States had a higher risk of SOT-related TB.
Treatment of latent infection in a SOT candidate is important but can be delayed. In two large cohorts of liver transplant patients, treatment of latent infection was associated with fewer cases of active TB among those treated compared with those not treated. The advantage of treated latent infection before SOT includes a higher efficacy in the absence of immunosuppression with fewer drug interactions, lower medication burden, and better tolerance. However, there may not be adequate time for treatment before transplant, drug-induced liver disease may be difficult to distinguish from underlying liver disease, and drug-induced injury could prompt fatal injury or require emergent liver transplant. Many institutions wait to treat latent infection soon after SOT during the period at greatest risk for reactivation (discussed earlier). The disadvantages to this practice include the difficulty in discerning drug-induced liver toxicity from graft rejection (in the case of liver transplant), jeopardizing injury to a new liver graft, high pill burden, managing drug interactions, and the theoretically less effective treatment during immunpsuppression. Among the first-line latent infection regimens mentioned earlier, RIF or rifapentine/INH use is likely a better option before transplant given the shortened duration and is less attractive after transplant owing to the increased incidence of drug-drug interactions with immunosuppressive medications, respectively. The INH regimen is more likely to be a better option after transplant as it has fewer drug-drug interactions. Any decision not to treat latent infection should be discussed with the patient and medical team weighing all risks and benefits.
TB screening among donors plays a key role in prevention of SOT-related TB given that donor-derived transplanted allografts result in both pulmonary (e.g., lung donor) and extrapulmonary (non–lung donor) TB. For obvious reasons, only limited information regarding TB risk is available in deceased donors. Although some committees have endorsed the use of IGRA or TST (if time permits) when a potential donor is identified, to date there is insufficient evidence on the sensitivity and specificity of these tests on dying or deceased donors. That said, a positive result should prompt further workup for active TB, as such a donor should be excluded for transplant. Some have suggested that chest radiography or CT may play a role in identifying patterns consistent with TB. A thorough review of donor risk factors (perhaps from family members) should be performed and considered, including homelessness or recent incarceration, travel or residence in a high TB-endemic area, history of TB, alcoholism, history of recurrent pneumonia, and so on. , For donors with probable latent infection, most experts suggest that the recipient should be treated for latent infection as the lungs carry the highest risk of donor-derived TB.
Living donors should be tested in a fashion similar to SOT candidates/recipients, especially as the diagnosis of active TB is a contraindication for organ donation. In the United States, all living donors have a routine chest radiograph and those with a risk for TB also have testing with either IGRA or TST. Symptoms consistent with TB or concerning chest radiographic findings should prompt a workup for active TB. In a European survey of transplant centers, 48% of centers used TST, 30% used IGRA alone, and 16% used both, demonstrating the variety of approaches. More rigorous screening recommendations have also been endorsed that include a careful epidemiologic and personal medical history, physical examination and chest radiography (regardless of TST/IGRA results), both IGRA and TST testing (with TST booster if no recent testing has been performed and IGRA especially in BCG-vaccinated patients), bronchoscopic testing for mycobacterial growth before lung donation, and urinalysis with microscopy or genitourinary testing for donors living in intermediate- or high-risk countries before kidney transplant donation. For donors with latent infection, treatment should be offered to the donor. The risks of delaying transplant if no other donor is available and the benefits of treatment (and risk of drug interactions) should be individualized as treatment need not be completed before transplant. As many as 30% to 40% of organ donors in TB-endemic countries are recognized to have latent infection and require treatment before transplant would significantly reduce the donor pool. Some institutions with high TB-endemic rates treat the recipient with INH without donor screening, assuming that this regimen after transplant will prevent reactivation, donor transmission, and de novo infection during the time of maximal immunosuppression.
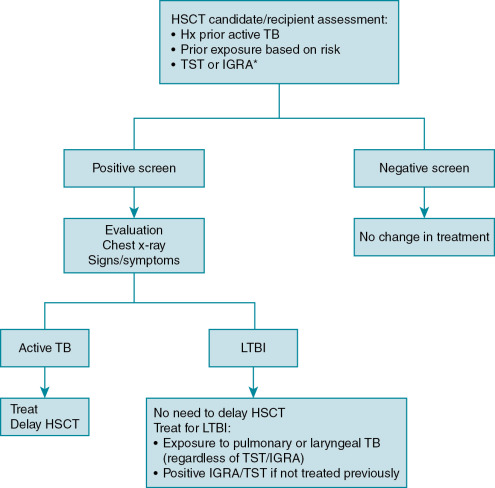
There are some distinctions between TB evaluation among HSCT candidates. For example, there are no formal recommendations to screen HSCT donors, as the risk of transmission is rare. , Donors with active TB should undergo treatment and avoid donation. However, most experts believe that transplanting HSCT from an untreated, latently infected donor poses no risk to the recipient. There is no consensus regarding screening practices for HSCT candidates. A survey of HSCT centers in Europe reported that only 10% of centers systematically screen for TB and only 51% would screen once clinical suspicion arose. Some guidelines recommend that candidates be assessed by history for TB risk factors and the extent of the evaluation after that is somewhat controversial. Certain guidelines dissuade the use of TST, suggesting that IGRA may be more useful. Anyone with TB risk factors (e.g., prior TB exposure, history of active TB, or positive TST/IGRA) should undergo evaluation for active TB or latent infection ( Fig. 16.2 ). Active TB disease should warrant treatment with delay of HSCT. The timing of when to start the HSCT after initiating anti-TB drugs should be individualized to the patient and involve discussion with the patient, infectious disease consultant, and primary HSCT team. Latent infection does not require delay in HSCT but should be treated based on a positive TST/IGRA result or recent exposure to someone with high-risk active TB (e.g., smear-positive sputum with pulmonary or laryngeal TB) regardless of the TST/IGRA result. Interestingly, two large studies in Korea, a high TB-endemic country, demonstrated that INH treatment for latent infection before HSCT (defined by IGRA positive result) did not reduce the incidence of TB, although this may have been due to TB cases that occurred from reinfection after HSCT. Although there is no question that TB in HSCT recipients is an important infectious complication, the role of TB screening and its benefits are less defined.