Investigation
Number of subjects
Age (years)
Iodine deficient
Prevalence (%)
Country
Laurberg et al. (1998) [4]
423
100
68
66–70
Yes
No
12 (female)
2 (female)
Denmark
Laurberg et al. (2006) [9]
4649
40–45
60–65
Yes
15
25–30
Denmark
Karger et al. (2010) [6]
736
Adult woman
Yes
45
Germany
Aghini-Lombardi et al. (2013) [12]
1411
Adult
Yes
No
46
26
Italy
A Danish investigation, the DanThy study, evaluated thyroid function before and after the mandatory iodine fortification of table salt and salt in bread in Denmark [9, 10]. Prior to the introduction of iodine, the prevalence of thyroid nodules >1 cm increased with age from 15% in wo me n aged 40–45, to 25–30% in those aged 60–65 [9]. Eleven years after initiation of the iodization requirement, the DanThyr study showed that among the 618 subjects with thyroid nodules at baseline, 24% had no evidence of nodules in follow-up [10] indicating reversal of nodular disease with normalization of iodine levels. In a survey of 1411 subjects in Pescopagano, an iodine-deficient village in Southern Italy, goiter prevalence was 16% in children and 59.8% in adults [11]. Dietary iodinated salt incorporation decreased the overall prevalence of goiter from 46%, in 1995, to 26%, in 2010 (P < 0.0001) [12]. Similar changes were noted in an iodine-deficient region of China where schoolchildren aged 8–10 years showed a reduction in goiter prevalence pre- and post-normalization of iodine levels from 18% to 9%, respectively [13]. Iodine repletion leading to the correction of thyroid disorders has been consistent in other studies [14–16]. Table 1.1 shows the prevalence of goiter/thyroid nodules in iodine deficient compared to iodine sufficient areas. This change is not permanent though; if iodized salts are removed from the diet and iodine levels become once again insufficient, goiter prevalence rapidly returns to pre-iodization levels [17].
Radiation exposure, thyroid nodularity, and cancer risk are correlated. Antonelli et al. in Pisa, Italy, evaluated 50 male medical workers occupationally exposed to radiation and compared them to nonexposed workers. Thyroid nodules were detected in 38% of exposed workers compared to 13% of those unexposed, living in the same area [18]. Trerotoli et al. in a cross-sectional study in Bari, Italy, evaluated the prevalence of nodules in an area of mild iodine deficiency in 304 people in work-related “maximum risk category for radiation exposure.” Nodules >1 cm were detected in 19% of nonexposed males to radiation compared to 4–9% of exposed males depending on the degree of radiation exposure [19]. The authors concluded that occupational exposure to radiation and iodine deficiency did not correlate with increased nodule risk and the higher numbers of nodules in the nonexposed group was related to this group having a stronger family history of thyroid disease. A similar study evaluated 1247 cleanup workers from Estonia sent to Chernobyl in 1986 for an average of 3 months soon after the nuclear plant accident. The incidence of thyroid nodules measured by ultrasound 8 years post exposure was 10.2%. The authors concluded that Chernobyl cleanup workers from Estonia did not experience an increased risk of nodular thyroid disease associated with exposure to external radiation [20]. These studies suggest that mild to moderate exposure to radiation does not significantly increase risk of nodule formation.
In a more intense radiation exposure setting, Takahashi et al. [21] evaluated 815 subjects present during the atomic bomb test, BRAVO on March 1, 1954, at Bikini Atoll, Marshall Islands. Their goal was to establish the prevalence of thyroid nodules attributed to fallout radiation. Of the 33% with thyroid nodules, cancer prevalence was particularly high (3.2%) in women who were aged 1–10 at the time of detonation. Papillary type thyroid carcinoma is the most frequently encountered malignancy (92%) in the Marshallese born prior to the 1954 detonation [22]. It is estimated that an overall 20% of Marshallese will develop thyroid cancer as a result of radiation fallout, with the highest estimated lifetime risk of 95% from those from Rongelap Island and Ailinginae community due to these areas having the highest fallout [23].
Suzuki et al. evaluated those under 18 years of age living in Fuskushima, Japan, during the 2011 nuclear power plant accident . While the prevalence of thyroid nodules was not very high (2108 of 300,476 screened), the overall prevalence of thyroid cancer was determined to be 37.3 per 100,000 [24]. This high rate of cancer is thought to be due to the use of highly sophisticated ultrasound techniques rather than a true increase due to radiation exposure [24, 25]. A cohort study survey of 4091 survivors of the Hiroshima and Nagasaki atomic bomb blast of 1945 were evaluated 55–58 years post exposure [26]. Thyroid disease was identified in 45% of participants with the prevalence of solid nodules, malignant tumors, benign nodules, and cysts of 15%, 2%, 5%, and 8%, respectively. A significant linear dose-response relationship was observed for the prevalence of all solid nodules, malignant tumors, benign nodules, and cysts (P < 0.001) in those exposed to radiation from the nuclear accident [26].
A cross-sectional analysis attempting to link an association between type 2 diabetes mellitus and thyroid nodules in Zhejiang Province, China, observed a thyroid nodule incidence rate of 81.4% in diabetic patients and 70.7% in nondiabetics. They concluded that though there was a higher incidence of thyroid nodules in the study group, diabetes was not a risk factor for thyroid nodules [27]. This is in contrast to a single-center, prospective case-controlled study in Turkey where 51% of prediabetics and 62% of diabetics possessed thyroid nodules compared to 24% of nondiabetics [28]. The authors suggest an association between impaired glucose metabolism and thyroid nodule prevalence. A possible cause of the difference between the two groups could be that the latter group was studied in a mild-to-moderate iodine-deficient area of Turkey while the iodine status of the first group in China was not revealed.
Volzke et al., in a cross-sectional study of 3662 subjects, evaluated the association between insulin-like growth factor-1 (IGF-1 ) and goiter risk in those without a history of thyroid disorders [29]. Those with serum IGF-1 levels in the upper tertile had a higher odds for goiter development relative to subjects with serum IGF-1 in the lower tertile [odds ratio (OR) 1.67, 95% confidence interval (CI) 1.24–2.26 in women, OR 2.04, 95% CI 1.55–2.68 in men]. Patients treated with growth hormone (GH) , for GH deficiency, developed a high (27%) incidence of thyroid nodules with the primary predictor of nodule development being serum IGF-1 levels (P = 0.038) [30]. Patients with active acromegaly, a condition with elevated endogenously secreted GH and subsequent high IGF-1 levels, were shown to have increased thyroid volume of up to 20% [31]. Thyroid volume was reduced with the normalization of IGF-1 by 21.5% in medically controlled (P < 0.005) and 24.2% in surgically cured (P < 0.002) acromegalic patients [31].
An association between thyroid nodularity and uterine fibroids was described in 925 women of whom 18% had coexisting thyroid nodules with uterine fibroids. A significant association was noted between both fibroids and thyroid nodules (P = 0.01) with a closer relationship in premenopausal women (n = 445, P = 0.001). This suggests that the age and presence of uterine fibroids are independent risk factors for the presence of thyroid nodules [32]. Further, systemic estradiol (E2) levels in premenopausal women showed an inverse correlation with the incidence of thyroid nodules (P = 0.024; OR = 0.631; CI, 0.424–0.940) [32].
A cross-sectional study evaluated the association between alcohol consumption and thyroid nodular disease , concluding that increasing levels of alcohol consumption are associated with a lower prevalence of thyroid enlargement as well as a lower prevalence of solitary thyroid nodules [33].
The association between smoking and goiter formation tends to vary depending on the region of the study. In iodine-sufficient areas, smoking tends not to have an impact on thyroid volume or nodular formation as noted in a study in the iodine-sufficient city of Istanbul [34], while, in iodine-insufficient areas such as Copenhagen and Aalborg, Denmark, smokers tend to have thyroid enlargement (OR, 2.9; 95% CI, 2.2–3.7) and palpable goiters (OR, 3.1; 95% CI, 1.6–5.8) compared to ex-smokers and those who never smoked [35]. The fraction of goiter cases attributable to smoking was 49% (95% CI, 29–65%) [35]. This has been shown in another study where smokers had a 30% incidence of goiter compared to 3% of nonsmokers [36].
In an evaluation to better understand the correlation between smoking, iodine status, and nodule formation, Vejbjerg et al. [37] evaluated goiter prevalence before and after mandatory salt iodization in Denmark in 2000. The overall difference in thyroid volume between heavy smokers and nonsmokers was significantly reduced after iodization of salt from 24% to 12%, respectively. A study in Pomerania, Germany, supported the declining risk of goiter formation with better iodine intake in smokers [38]. It is thought that thiocyanate, a competitive inhibitor of iodine uptake, may be the goitrogenic substance from tobacco smoke [39, 40]. Thiocyanate is also found in polluted environments and thought to possess concentration-dependent antithyroid properties [40–42]. The Endocrine Society has recently reported on environmental endocrine-disrupting chemicals [43].
Palpation (Table 1.2)
As the least sensitive method of detecting nodules , palpation identifies the prevalence of nodules of around 4–7% [44–48]. Palpation is a more reliable technique when nodules are greater than 1 cm [49]. Palpation carries a high false-positive rate where up to 68% of palpable nodules may not be identified on high-resolution ultrasound [47]. Palpable nodules appear to be more frequently detected in females (6%) than males (2%) [50]. In a 15-year follow-up study by Vander et al., the incidence of new nodules was 2% in females and 1% in males, with an overall annual incidence rate, by palpation, of 0.1% [50]. A Scandinavian study of a middle-aged woman found a 6.5% prevalence of palpable nodules in females [51]. The Whickham survey in 1977 [52], conducted in Whickham, England, evaluated 2779 people (82% of the available population) and reported that 8.6% of the study group had palpable nodules not visible to the eye and 6.9% had palpable and obviously visible nodules with a higher prevalence in women (5.3%) than men (0.8%).
Table 1.2
Comparison between prevalence of thyroid nodules by palpation versus ultrasound technique
Investigation | Number of subjects | Age (years) | Prevalence by palpation | Prevalence by ultrasound | Country |
|---|---|---|---|---|---|
Wiest et al. (1998) [47] | 2441 | 20–72 | 6.9% | 10.2% | Estonia |
Ezzat et al. (1994) [66] | 100 | Adult | 21% | 66% | United States |
Bruneton et al. (1994) [67] | 1000 | Adult | 34.7% | France | |
Tunbridge et al. (1977) [52] | 1977 | Adult | 8.6% | United Kingdom |
To compare the accuracy of clinical palpation to diagnose solitary thyroid nodules to ultrasound, Tan et al. noted that the majority of palpable nodules (89%) are greater than 1 cm and around 50% of presumed solitary nodules on palpation turned out to be multinodular by ultrasound [49]. A similar study, evaluating the accuracy of palpation compared to ultrasound showed that, 6.9% of nodules were identified by palpation, while missing 3.3% when subjects were re-evaluated by high-resolution ultrasound (total of 10.2% by ultrasound) [47]. Interestingly, high-resolution ultrasound did not confirm the existence of 68% of nodules found by physical exam, while physical exam missed 79% of nodules detected on ultrasound [47] showing the poor sensitivity and specificity of palpation methods. A systematic review of studies from non-endemic goiter areas found the prevalence of thyroid nodules detected by palpation ranging from 4.7 to 51.0 per 1000 adults and 2.2 to 14.0 per 1000 children [53].
Children have a lower, 0.05–1.8%, prevalence of thyroid nodules [54, 55]. In an evaluation of 4819 school-aged children between 11 and 18 years of age, 4.6/1000 had thyroid nodules. Reevaluation of the same subjects 20 years later noted an increase to 23.2/1000 [56] suggesting an increase in prevalence with age. Though the incidence of thyroid nodules is fairly uncommon in the pediatric age group, it must be noted that the rate of cancer is higher than that seen in adults [57–59] with an overall 18.7–26.4% risk of malignancy in children [54, 60, 61] .
Ultrasound (Table 1.2)
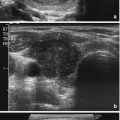
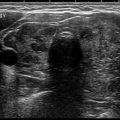
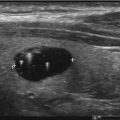
When comparing the accuracy of thyroid ultrasound to postmortem pathological review, ultrasound has a sensitivity of 89% and a specificity of 84% [62]. High-resolution ultrasound (US) machines are able to detect impalpable nodules as small as 1–3 mm in size [63]. The reported prevalence of thyroid nodules by ultrasound is 20–70% in the general population [64–67] with an increase in prevalence with age, peaking in the seventh decade (P < 0.001), with a higher prevalence in women than men [68]. There is a 1.6% annual risk for multinodularity (OR, 1.02; P < 0.001) in contrast to a decreasing risk of malignancy with age from 22.9% aged 20–30 years to 12.6% aged >70 years (OR 0.972; P < 0.001) [69]. The prevalence of thyroid nodules appear to be similar in different areas around the world [70–72] with iodine status remaining as the main determining factor of prevalence differences [4–6].
Other Imaging
Computed tomography (CT), magnetic resonance imaging (MRI) , carotid duplex scanning, and 2-18[F] fluoro-2-deoxy-d-glucose (FDG) positron emission tomography (PET) are used in medicine with variable frequencies. Since they are rarely used specifically to evaluate the thyroid gland, the discovery of thyroid lesions is typically considered incidental. Another chapter describing incidental thyroid nodules can be found in this text, and we refer you to that chapter for details regarding incidental thyroid nodules by another imaging modality.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree