Abstract
Endometriosis is a complex syndrome characterized by an estrogen-dependent chronic inflammatory process that primarily affects pelvic tissues, including ovaries, and results from repetitious ovulatory menstrual episodes and retrograde travel and survival of shed endometrial tissue in the lower abdominal cavity. It is the most common cause of chronic pelvic pain in women and is linked with infertility. The key underlying mechanisms affect the intracavitary endometrium and (ectopic) endometriosis tissue; these include poorly differentiated endometrial mesenchymal progenitor/stem cells, widespread epigenetic defects, estrogen receptor-β-mediated activation of inflammation, and progesterone resistance. Pain is managed by the suppression of ovulatory menses and estrogen production, cyclooxygenase inhibitors, and the surgical removal of endometriosis, whereas in vitro fertilization is frequently used to enhance fertility.
Keywords
Endometriosis, chronic inflammation, endometrium, progenitor/stem cells, peritoneum, epigenetic defect, estrogen biosynthesis, estrogen receptor-β, progesterone resistance, pelvic pain, infertility, oral contraceptive, GnRH analogue, aromatase inhibitor, laparoscopy
Introduction
- ◆
Severe primary dysmenorrhea, repeated episodes of ovulation and retrograde menstruation, and pelvic endometriosis visualized by laparoscopy probably represent the early and progressive stages of the same disease process.
- ◆
An average patient with endometriosis suffers from its symptoms for decades. Severe menstrual pelvic pain initially experienced during the teenage years gradually evolves in quality and severity as the inflammatory stimuli from pelvic disease persist and the peripheral and central nervous system is continually re-conditioned, leading to the phenomenon of central sensitization.
- ◆
The symptoms of endometriosis often disrupt the social, professional, academic, and economic potential of young women. Living with severe cyclic or continuous pelvic pain or the threat of its return, often for decades, can lead to anxiety and depression. Thus, the current and narrow anatomic definition of endometriosis neither reflects its true complexity and chronic nature, nor addresses the potentially grim course of this disease in the absence of appropriate medical and surgical management.
Defining Endometriosis
The term “endometriosis” has been conventionally described as the presence of endometrial tissue outside of the uterine cavity, primarily on pelvic tissues and ovaries. However, this narrow anatomic definition is not sufficient to explain its natural history and duration, the full spectrum of its clinical features, the frequent recurrence of its symptoms, the underlying molecular pathophysiology, or its responsiveness to currently available management strategies. Unfortunately, the anatomic definition can lead to confusion about treatment and even denial of hormonal or surgical treatment to endometriosis patients who do not have recognizable pelvic peritoneal lesions at the time of laparoscopy. Thus the classical definition of endometriosis needs to be modernized to a more patient-focused version that recognizes the cellular and molecular origins of the disease, its natural history from teenage years to menopause, its complex, chronic, and systemic nature, the variety of tissues involved including the central nervous system, and the need for long-term management.
Many clinicians, patients, and researchers now recognize endometriosis as a complex clinical syndrome characterized by an estrogen-dependent chronic inflammatory process that affects primarily pelvic tissues, including ovaries, and is strongly linked to recurrent pelvic pain and persistent episodes of ovulation, menstruation, and cycling steroid hormones. Endometriosis is the most common cause of chronic pelvic pain in reproductive-age women. It is also strongly associated with infertility.
In addition to common pelvic endometriosis (affecting pelvic peritoneal surfaces, subperitoneal fat and ovaries), which comprises the vast majority of all cases of endometriosis, the disease may also affect the bladder, bowel (most commonly the rectum and appendix), deep pelvic nerves, ureters, anterior abdominal wall, abdominal skin, diaphragm, pleura, lungs, and the brain. Thus, clinical findings can vary widely and include hematuria, hematochezia, pneumothorax, and seizures. The cyclic nature of these otherwise inexplicable symptoms and their association with menstruation (i.e., catamenial) are suggestive of endometriosis as an underlying etiology.
The symptoms of endometriosis often disrupt the social, professional, academic, and economic potential of young women. Living with severe cyclic or continuous pelvic pain or the threat of its return, often for decades, can lead to anxiety and depression. Here, we will summarize the salient features of endometriosis, followed by a comprehensive definition of the disease process and the clinical and molecular advances that have been made over the past decade.
Key Clinical Observations
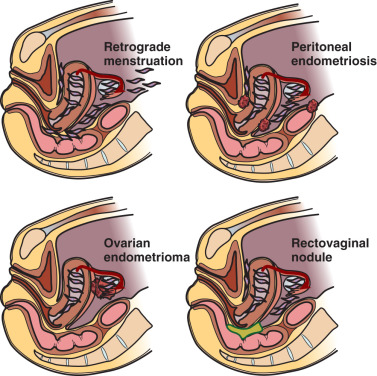
It is important for clinicians and researchers to develop a 360-degree perspective of endometriosis and take approaches to its diagnosis and treatment that will maximize the benefit to the patient suffering from this disease. The following observations or postulates must be recognized and placed in a broader and unified pathological context. (1) Severe primary dysmenorrhea, repeated episodes of ovulation and retrograde menstruation and pelvic endometriosis visualized by laparoscopy probably represent the early and progressive stages of the same disease process ( Fig. 25.1 ). (2) An average patient with endometriosis suffers from its symptoms for decades. Severe menstrual pelvic pain initially experienced during the teenage years gradually evolves in quality and severity as the inflammatory stimuli from pelvic disease persist and the peripheral and central nervous system is continually re-conditioned, leading to the phenomenon of central sensitization ( Fig. 25.2 ). (3) Retrograde menstruation associated with ovulatory cycles is the primary source of pelvic peritoneal endometriotic implants, rectovaginal nodules, as well as ovarian endometriomas, which arise primarily from cystic corpora lutea harboring menstrual tissue ( Figs. 25.3 and 25.4 ). (4) In the absence of any visible pelvic endometriotic implants, microscopic inflammatory processes in the pelvic peritoneum or eutopic endometrial tissue can exist and cause persistent pain that responds to treatments that suppress ovulation and estrogen production. (5) Newly diagnosed patients with endometriosis significantly benefit from surgical or medical treatment; almost all treatment-naïve patients achieve pain relief. However, the response rate progressively and sharply decreases with each repeated treatment. Thus it is more challenging to treat recurrent disease or its symptoms. (6) Any successful management strategy of endometriosis-associated pelvic pain should include long-term suppression of ovulatory menses.
Key Experimental Observations
It is also critical for clinicians to understand the following key mechanistic, cellular, and molecular findings that underlie endometriosis.
(1) Ovulatory menstruation is inextricably linked to the development of endometriosis, at least in its early stages ( Fig. 25.5 ). Species that do not menstruate do not develop endometriosis, and women who never had ovulatory menses do not develop endometriosis. Disruption of ovulatory menses in women with endometriosis is usually therapeutic (see Fig. 25.5 ). (2) Endometriosis involves multiple tissues. Distinct cellular and molecular abnormalities involving inflammation and steroid responsiveness have been well described at least in two types of tissues: eutopic (intracavitary) endometrium and ectopic endometriotic tissue. It is possible that the list of affected tissues will grow. Thus, surgical removal of ectopic pelvic endometriotic tissue, even by the most capable surgeons, will not cure endometriosis. (3) Histologically, the majority of endometriotic implants are primarily composed of stromal cells and contain a small epithelial component that lacks the deep invaginations (glands) observed in eutopic endometrial tissue (see Fig. 25.4 ). The endometriotic stromal cell is epigenetically abnormal and demonstrates partial phenotypes of ovarian theca-granulosa cells and macrophages. Endometriotic stromal cells express the full cascade of steroidogenic enzymes such as aromatase and produce remarkable quantities of progesterone and estradiol from cholesterol. The endometriotic stromal cell also produces large amounts of immunologically active substances, such as interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF), regulated upon activation normal T cell expressed and secreted (RANTES), and monocyte chemoattractant protein-1 (MCP-1). (4) The magnitude of the role of estrogen in endometriosis is somewhat similar to the role of insulin in diabetes. Estradiol is a master regulator of all key pathological processes in endometriosis and enhances lesion survival and inflammation leading to pain (see Fig. 25.5 ). In the absence of estradiol, endometrial tissue attachment to peritoneum, lesion survival, production of inflammatory substances (e.g., metalloproteinases, cytokines or prostaglandins, and growth factors), and angiogenesis do not occur. Estradiol, primarily acting via estrogen receptor-β (ERβ), triggers pathways to enhance lesion survival, remodel pelvic peritoneal tissue, and produce inflammatory substances, which stimulate nociceptors in pelvic tissues and trigger pain (see Figs. 25.2 and 25.5 ).
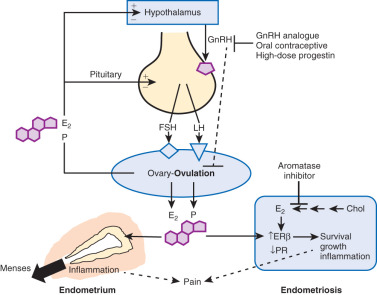
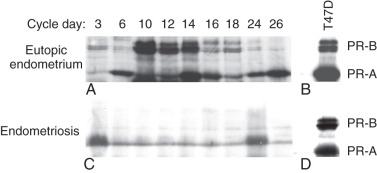
The denial of estradiol to endometriosis either naturally (menopause) or pharmacologically (ovarian suppression or aromatase inhibition) causes regression of the disease and its symptoms (see Fig. 25.5 ). (5) Endometriosis is resistant to the effects of progesterone because of a deficiency of progesterone receptor (PR) in this tissue ( Fig. 25.6 ). In fact, endometriotic tissue produces large amounts of progesterone locally, which is consistent with PR deficiency or progesterone resistance. This explains, in part, why endometriotic tissue responds poorly to progesterone. On the other hand, synthetic antiprogestin compounds, such as mifepristone and ulipristal acetate, which bind to low levels of PR with higher affinity, are therapeutic in endometriosis. The beneficial effects of synthetic progestins are probably mediated primarily via ovarian suppression.
Epidemiology
- ◆
Endometriosis affects an estimated 1 in 10 women during their reproductive years (i.e., usually between the ages of 12 to 52), or approximately 200 million women worldwide.
- ◆
The risk of endometriosis appears to increase with a greater lifetime number of ovulatory cycles. It is more relevant to discuss the prevalence of endometriosis rather than its incidence because the disease can go undiagnosed for decades. The underlying disease process can start as early as menarche, and last as long as four decades.
- ◆
Endometriosis is the most common pelvic pathology found in women undergoing laparoscopy or laparotomy for chronic pelvic pain or infertility.
Endometriosis affects an estimated 1 in 10 women during their reproductive years (i.e., usually between the ages of 12 and 52), or approximately 200 million women worldwide. However, it is more relevant to discuss the prevalence of endometriosis rather than its incidence because the disease can go undiagnosed for decades. The underlying disease process can start as early as menarche, and last as long as four decades. Though endometriosis may be suppressed via the inhibition of ovulation and menses, and pain or infertility may be managed by conservative surgical resection, the majority of these patients will not be cured of this disease. Endometriosis is the most common pelvic pathology found in women undergoing laparoscopy or laparotomy for chronic pelvic pain or infertility.
Retrograde menstruation is the most likely pathogenic mechanism for the development of endometriosis ( Fig. 25.3 ). The likelihood of implantation of retrograde-traveled endometrium may be influenced by the recent major increase in the number of menstruations due to earlier ages of menarche and progressively decreasing number of pregnancies per woman that naturally disrupt ovulatory cycles. Indeed, the risk of endometriosis appears to increase with a greater lifetime number of ovulatory cycles. Moreover, ovulation and the genesis of ovarian endometriosis has been linked, as endometriomas seem to develop from follicles immediately after ovulation and by direct transition from hemorrhagic corpora lutea ( Fig. 25.3 ).
Pathology
- ◆
Retrograde menstruation associated with ovulatory cycles is the primary source of pelvic peritoneal endometriotic implants, rectovaginal nodules, and ovarian endometriomas.
- ◆
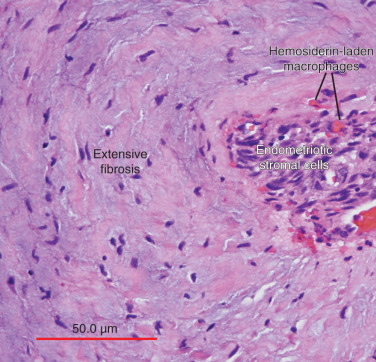
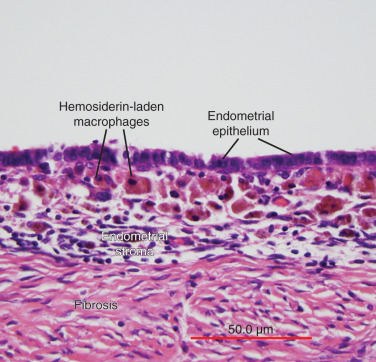
Histologically, the majority of endometriotic implants are composed primarily of stromal cells and contain a small epithelial component that lacks the deep invaginations (glands) observed in eutopic endometrial tissue. Episodes of bleeding and remodeling in adjacent tissues including the peritoneum, adipose tissue and ovaries give rise to remarkable inflammation and fibrosis.
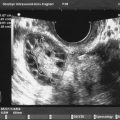
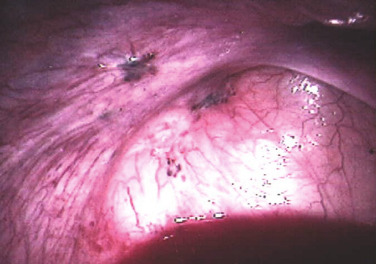
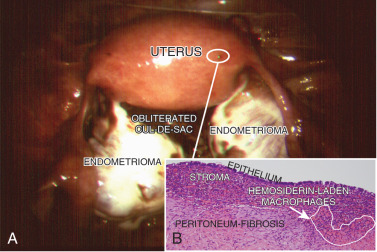
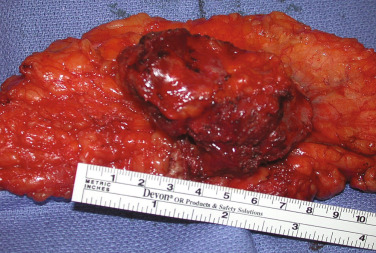
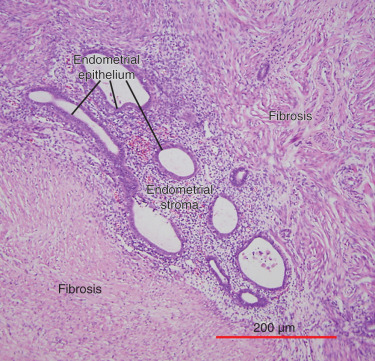
The term endometriosis initially referred to a histologic diagnosis, but has eventually evolved to describe a clinical syndrome as the clinical picture became clearer, expanding to include symptoms, laparoscopic findings, and molecular discoveries. Histologically, endometriosis refers to the presence of endometrium-like tissue outside of the uterine cavity, usually on the pelvic peritoneal surfaces or in the ovary. Histologic diagnosis is made upon the identification of three key elements: (1) endometrial stromal cells, (2) endometrial epithelial (glandular) cells, and (3) evidence of chronic bleeding in or adjacent to endometrium-like tissue, including sheets of red blood cells and blood pigment (hemosiderin)-containing macrophages. A pathologist usually requires two of these three findings to make a histologic diagnosis of endometriosis. Fibrosis comprising fibroblasts and extracellular matrix is commonly observed surrounding endometriotic implants. It is not uncommon to receive a pathologic diagnosis stating, “fibrotic tissue consistent with endometriosis.” In this case, the pathologist probably found only endometrial stromal-like cells and fibrosis but not endometrial epithelial cells. Pelvic endometriosis is commonly classified by three common locations: (1) peritoneal endometriosis found on uterine serosa or pelvic peritoneal or subperitoneal tissue ( Figs. 25.7 and 25.8 ); (2) rectovaginal nodule ( Fig. 25.9 ); and (3) ovarian endometrioma ( Fig. 25.10 ).
Pathophysiology
- ◆
Ovulatory menstruation is inextricably linked to the development of endometriosis, at least in its early stages.
- ◆
The key underlying mechanisms affect the intracavitary endometrium and extra-uterine endometriosis tissue; these include poorly differentiated endometrial mesenchymal progenitor/stem cells, widespread epigenetic defects, estrogen receptor-β-mediated activation of inflammation, and progesterone resistance.
- ◆
The endometriotic stromal cell is epigenetically abnormal and demonstrates partial phenotypes of ovarian theca-granulosa cells (estradiol biosynthesis) and macrophages (cytokine production). Estradiol, primarily acting via estrogen receptor-β, is a master regulator of all key pathological processes in endometriosis and enhances lesion survival and inflammation leading to pain.
- ◆
Endometriosis is resistant to the effects of progesterone because of a deficiency of progesterone receptor (PR) in this tissue, leading to differentiation defects both in endometrium and endometriosis.
Cellular Origins of Endometriosis
A number of mechanisms have been proposed regarding the histologic origins of endometriosis. Sampson and colleagues postulated that fragments of menstrual endometrium pass retrograde through the fallopian tubes, then implant and persist on peritoneal surfaces. This has been demonstrated in primate models and observed naturally in humans; it is also supported by the observation that spontaneous endometriosis occurs exclusively in species that menstruate; that is, female primates including humans. The alternative coelomic-metaplasia hypothesis describes the genesis of endometriotic lesions within the peritoneal cavity via differentiation of mesothelial cells into endometrium-like tissue. A third hypothesis argues that menstrual tissue from the endometrial cavity reaches other body sites via veins or lymphatic vessels. Additionally, it has been proposed that circulating blood cells originating from bone marrow differentiate into endometriotic tissue at various body sites. The difficulty in constructing clinically relevant models made it challenging to demonstrate or refute the potential mechanisms proposed as alternatives to Sampson’s retrograde menstruation hypothesis.
Eutopic Endometrium
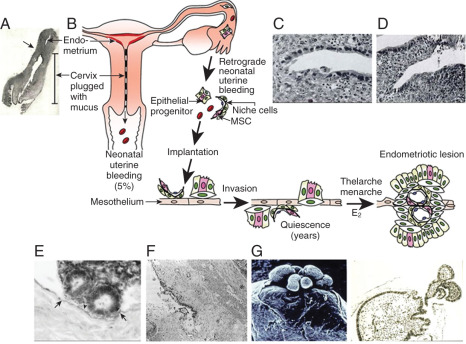
Sampson’s hypothesis of implantation of intracavitary endometrial tissue offers a plausible mechanism for the majority of abdominal endometriotic lesions, but does not explain why only some women develop endometriosis ( Fig. 25.11 ). Although almost all women have reflux menstruation of endometrial tissue mixed with blood into the peritoneal cavity, endometriosis is encountered in only approximately 10% of cases (see Fig. 25.11 ). Two potential mechanisms may explain the successful implantation of refluxed endometrium to the peritoneal surface. First, eutopic endometrium or the tubal mucosa of women with endometriosis qualitatively exhibits multiple subtle but significant molecular abnormalities, including activation of oncogenic pathways or biosynthetic cascades that favor increased production of estrogen, cytokines, prostaglandins, and metalloproteinases. When this biologically distinct tissue attaches to mesothelial cells, the magnitude of these abnormalities is drastically amplified to enhance implant survival. Second, larger quantities of otherwise normal endometrial tissue, including somatic stem cells, may arrive at the pelvic abdominal cavity during each menses in women with endometriosis compared with endometriosis-free women. It was also suggested that endometrial stem/progenitor cells may be involved in the pathogenesis of premenarchal and adolescent endometriosis through retrograde neonatal uterine bleeding due to maternal progesterone withdrawal at birth. Endometrial stem/progenitor cells would remain dormant beneath the peritoneum until increasing estradiol levels at menarche activate them to initiate clonal growths of ectopic endometrium and establish early onset endometriosis prior to menstruation ( Fig. 25.12 ).
It has also been proposed that a defective immune system might fail to clear implants off the peritoneal surface. However, this argument assumes that a healthy immune system should attack its native tissue, which is, at best, a variable phenomenon.
Molecular and Cellular Defects in Eutopic Endometrium
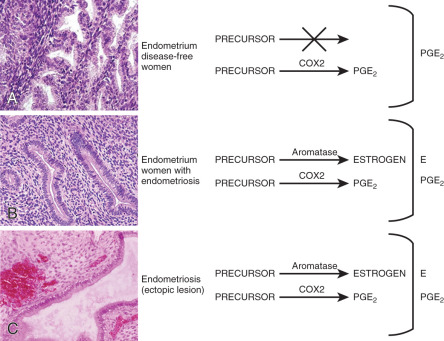
Histologically, eutopic endometrium of women with endometriosis looks identical to that of endometriosis-free women. However, a considerable body of clinical and molecular evidence supports the existence of qualitative and quantitative abnormalities in eutopic endometrial tissue of women with endometriosis. For example, stromal cells of eutopic endometrium of women with endometriosis contain strikingly higher levels of aromatase, cyclooxygenase (COX)2 and IL-6 mRNA and protein as well as higher levels of aromatase enzyme activity ( Fig. 25.13 ). Gene expression profiling of eutopic endometrium from women with or without endometriosis confirmed these earlier observations and found dysregulation of selected genes that contribute to implantation, including genes involved in embryonic attachment, embryo toxicity, immune dysfunction, and apoptotic responses, as well as genes that likely contribute to the pathogenesis of endometriosis, including aromatase, progesterone receptor, and angiogenic factors.
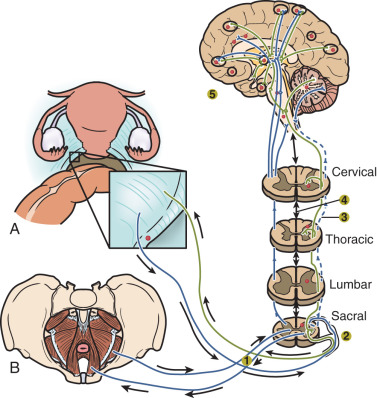
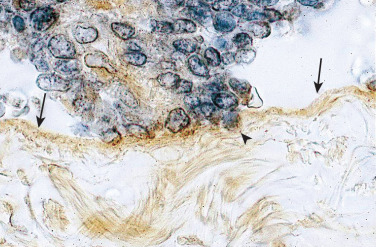
Small nerve fibers were detected in the functional layer of eutopic endometrial tissue in all women with endometriosis but not in the eutopic endometrium of disease-free women. This may have important implications for understanding the generation of pain in these patients. For example, this may explain why endometriosis patients continue to experience pain after surgical resection of ectopic implants. Moreover, the pathologic presence of nerve fibers points to a general defect in the differentiation of somatic stem cells in the endometrium of patients with endometriosis (see subsequent text).
Progenitor/Stem Cells
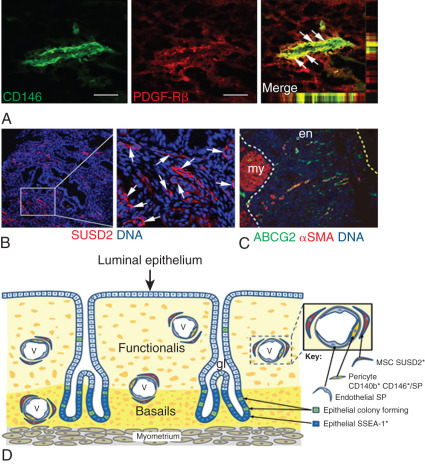
Mesenchymal stem/stromal cells have been isolated from endometrial tissue and characterized; they have similar properties to bone marrow mesenchymal stem cells. Selective markers for mesenchymal stem cell enrichment have been identified: CD146, platelet-derived growth factor receptor-β (PDGFRβ), and sushi domain containing-2 (SUSD2) reveal a perivascular location of mesenchymal stem/stromal cells (MSCs) and suggest a pericyte identity of these cells in endometrial basalis and functionalis vessels ( Fig. 25.14 ). Transcriptomics and secretomics of SUSD2 + cells confirm their perivascular phenotype ( Fig. 25.14 ). Stromal fibroblasts cultured from endometrial tissue or menstrual blood also have some mesenchymal stem cell characteristics and demonstrate broad differentiation potential for mesodermal, endodermal, and ectodermal lineages, indicating their plasticity. Recent molecular evidence suggests that mesenchymal stem cells of eutopic endometrium may give rise to endometriosis. Evidence has also suggested that bone marrow cells may contribute a small population of endometrial epithelial and stromal cells. The discovery of specific markers for endometrial stem/progenitor cells has enabled the examination of their role in endometriosis, adenomyosis, and healthy endometrial development for implantation.
Uterine Vascular Tissue
Menstruation occurs as a consequence of intense vasoconstriction and coagulation in the spiral arteries leading to intense inflammation and partial necrosis, after which the functionalis layer of the endometrium separates from the underlying basalis and is shed with blood ( Fig. 25.15 ). Based on the remarkable regenerative potential of the basalis of the endometrium during the next cycle, it has been suggested that endometriosis may originate from progenitor/stem cell populations that reside in this layer. After menstruation, the basalis layer rapidly regenerates under the influence of estrogen to build the functionalis within several days; thus, it is reasonable to assume that key tissue stem cell populations in the endometrium are concentrated in the basalis. It is also reasonable to hypothesize that the deeper the plane of separation between the basalis and functionalis layers, the higher the number of estrogen-responsive endometrial stem cells in the menstrual tissue that can reach the peritoneal cavity (see Fig. 25.15 ). Thus, one can envision that epigenetically defective cellular components in endometrial vessels involved in the menstruation process may also give rise to the endometriosis phenotype. This resonates with the clinical picture, as a history of heavy and prolonged menses is extremely common among women with endometriosis (see Fig. 25.15 ).
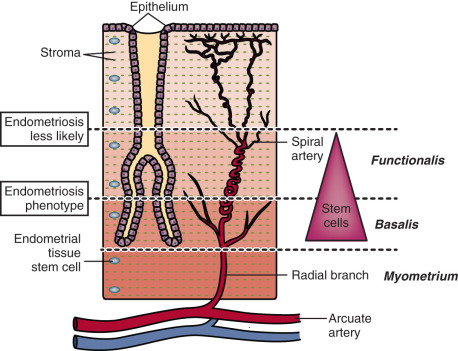
Peritoneum
For the establishment of peritoneal endometriosis, the adhesion of shed endometrial cells to peritoneal and subperitoneal surfaces involves the expression of extracellular membrane adhesion molecules and their coreceptors. Experimental findings and clinical observations also indicate that the process of implant attachment to the peritoneal surface and its eventual establishment in the subperitoneal space is quite rapid ( Figs. 25.11 and 25.12 ). Thus, a mesothelial surface with increased “receptivity” to the establishment of endometrial cells may contribute to the common phenotype of endometriosis.
An alternative hypothesis suggests that metaplasia of the coelomic epithelium is the origin of endometriosis. This theory is logical, because cells from both the peritoneum and endometrium derive from a common embryologic precursor: the coelomic cell. However, it is challenging to demonstrate this potential mechanism experimentally. Nonetheless, it is quite possible that the pelvic peritoneum and its mesothelial surface may play critical roles in the mechanism of endometriosis.
Key Biological Processes
The biology of endometriosis is primarily determined by the endometriotic stromal cell that dominates in quantity and exhibits the central pathologic processes in endometriotic lesions (see Figs. 25.4, and 25.8 to 25.10 ). The endometriotic stromal cell exhibits most of the key and clinically relevant molecular abnormalities, such as estradiol production, progesterone resistance, cytokine production, and prostaglandin production. However, it should be pointed out that the endometrial or endometriotic stromal cells can be readily maintained in primary culture to study their biology, whereas it is more challenging to culture epithelial cells. This limitation has led to a disproportionate number of mechanistic molecular studies employing endometrial or endometriotic stromal cells, and could have created a scientific bias against the potential roles of other cell types. Nevertheless, the following conclusions resonate with the existing data, the histologic sections of endometriotic implants, and the clinical observations.
Apoptosis
Apoptosis is clearly decreased in endometriotic stromal or epithelial cells compared with eutopic endometrial tissues. This may be linked to pathologic SF1 (estradiol biosynthesis) and ERβ expression. In endometriotic stromal cells, ERβ mediates antipoptotic effects of estradiol.
Differentiation
A central process in endometriosis is deficient differentiation (decidualization) of the stromal cell. This has been linked to progesterone resistance. Although the first molecular evidence of progesterone resistance was observed in the endometriotic epithelial cell, deficient stromal decidualization was eventually found to be the primary mechanism (see “ Progesterone Resistance ”). The notion of deficient differentiation also resonates with the proposed roles of epigenetically misprogrammed mesenchymal stem cells in endometriosis.
Inflammation
Inflammation is the most important pathologic process in endometriosis. It leads to pain, remodeling of neighboring tissues, fibrosis, adhesion formation, and infertility. The production of cytokines and prostaglandins, and the infiltration of immune cells are some of the hallmarks of inflammation. Interestingly, the endometriotic stromal cell is one of the major sources of cytokines and prostaglandins. Recurrent episodes of bleeding and an attempt of macrophages to remove the blood pigments also contribute to the inflammatory process and adhesion formation (see Fig. 25.4 ). Inflammatory processes are primarily induced by estradiol and mediated by ERβ in endometriosis.
Proliferation
The key cell type that proliferates extensively in endometrial tissue is the epithelial cell under the influence of estrogen. Most endometriotic implants, however, contain scant epithelium and are comprised primarily of stromal cells ( Figs. 25.4 and 25.8 to 25.10 ). Any human mesenchymal cell is not epigenetically programmed to proliferate extensively. Thus, as expected, there is limited proliferative activity in endometrial or endometriotic stromal cells in general. In fact, significantly lesser proliferative activity was reported in endometriotic tissue compared with eutopic endometrial tissue.
Invasion
Some invasive activity seems to be necessary for the initial attachment of endometrial tissue fragments to pelvic peritoneum and the establishment of subperitoneal endometriotic lesions. This is a complex process mediated by a number of molecular pathways. One of the best studied pathways involves high metalloproteinase activity in endometriotic implants. Either proliferation or invasion, however, contributes to a much lesser degree to the pathology of endometriosis compared with malignant (e.g., endometrial cancer) or benign (e.g., uterine leiomyoma) neoplasms.
Genetics
Inheritance
The risk of endometriosis seems to be transmitted in families in a polygenic manner. In one study, the mothers and sisters of women with severe endometriosis had a sevenfold higher likelihood of suffering from endometriosis compared with primary female relatives of their partners. Consistent with this observation is the finding that familial cases of endometriosis tend to be more severe and have an earlier onset of symptoms than sporadic cases. Based on a study of 3096 twins, the heritability of endometriosis, or the proportion of disease variance due to genetic factors, has been estimated at around 52%. These studies on familial endometriosis point to genetic transmission of the disease. The mode of transmission, however, seems to be complex, as seen in other chronic inflammatory conditions.
Candidate gene studies
A number of “off-the-shelf” candidate gene loci have been interrogated based on their biological plausibility and possible connection with endometriosis, but these studies were negative. Case-control genetic association studies performed primarily to find polymorphisms also failed to discover any function-altering germ-line mutations in familial endometriosis. Geneticists have cited a number of factors for the inability of candidate gene studies to ascertain the genetic basis of complex inflammatory diseases like endometriosis. The key reason for the lack of meaningful results, however, could be as simple as the actual absence of any classically defined germ cell mutations that cause the endometriosis phenotype.
Genetic Linkage Analysis
An international study group found evidence of significant linkage of endometriosis to chromosome 10q26 from the analysis of 1176 affected sister-pair families. Subsequent fine-mapping association analyses of 10q26 suggested a possible association of common variants near the CYP2C19 gene, but mutations that could explain the linkage signal could not be demonstrated. The same group later identified significant linkage to chromosome 7p13–15 in a sub-analysis of 248 families with more than 3 affected members, suggesting the presence of one or more rare variants conferring high risk; however, no mutations could be identified. As in the case of candidate gene studies, the genetic linkage analysis approach has not produced any meaningful results.
Genome-Wide Association Studies
Genome-wide association studies (GWAS) typically focus on associations between single-nucleotide polymorphisms (SNPs) and traits, such as major human diseases. Since 2010, a number of widely acknowledged endometriosis GWASs have been published; these studies employed datasets involving thousands of endometriosis cases and controls, primarily women of Japanese or of European ancestry. A meta-analysis of these GWAS datasets confirmed their findings and revealed additional loci.
Together, these studies have provided significant evidence for association of loci near: CDKN2B-AS1 (cyclin-dependent kinase inhibitor 2B antisense RNA), an intergenic region on chromosome 7p15.2, WNT4 (wingless-type MMTV integration site family member 4), VEZT (vezatin), an intergenic region on 2p14, and ID4 (inhibitor of DNA binding 4). Some of these loci had stronger effect sizes among stage III–IV cases, implying that they are likely to be implicated in the development of moderate to severe, or ovarian, disease. Despite these large-scale and expensive studies, no mutations affecting these genes in familial cases of endometriosis were reported. Further, no in vivo or in vitro functions of these genes or intergenic regions have been demonstrated in the pathogenesis of endometriosis to date.
Search for Somatic Mutations
As of July 2017, two whole-exome sequencing studies were published on the search for somatic alterations in ovarian endometriomas and nonovarian deep-infiltrating endometriosis. Li et al. first demonstrated a large number of single nucleotide variants in laser microdissected epithelial cells from ovarian endometriosis and matched eutopic endometrial epithelial cells and in endometrial epithelium from disease-free women. Most exomic single nucleotide variants were specifically present in both ectopic and eutopic endometrial cells and were associated with genes involved in cell adhesion and chromatin remodeling. These investigators did not demonstrate any functional, pathologic, or clinical consequences of these exomic variations.
A more recent exome-sequencing study using nonovarian deep-infiltrating benign endometriotic tissues showed low-frequency somatic mutations possibly affecting less than 20% of epithelial cells per lesion; no mutations were detected in stromal cells. Only five endometriotic tissues (26%) carried one of the 4 cancer-driver mutations including ARID1A and KRAS . A minority of epithelial cells (4% to 38%) in each lesion harbored a ARID1A or KRAS mutation. Since the risk of adenocarcinoma in nonovarian endometriosis is negligible, the clinical significance of these sporadic epithelial mutations are currently unknown.
Stromal cells comprise the majority of endometriotic lesions and account for steroid-related inflammatory processes but lack any somatic mutations. Taken together, unlike uterine fibroids, endometriotic implants do not seem to contain any clearly defined or distinct somatic mutations that are directly causative.
Epigenetic Defects
Epigenetic mechanisms refer to changes to the genome that do not involve a change in the DNA sequence. These mechanisms include DNA methylation and histone modification, each of which modifies how genes are expressed. These epigenetic influences may last through cell divisions for the duration of the cell’s life, and may also last for multiple generations.
Gene Expression
The failure to demonstrate causative germ cell or somatic mutations in endometriosis to date led researchers to turn their attention to epigenetic mechanisms. There are a number of general mechanisms responsible for gene expression in various cell types. First, certain genes can be expressed in a highly specialized cell type because a specific set of necessary signaling pathways and downstream transcription factors are selectively present in this particular cell. For example, the endometriotic stromal cell specifically expresses the prostaglandin (PG) E 2 receptor EP2 that activates the cAMP-dependent signaling pathway and its downstream effector steroidogenic factor-1 (SF1, or NR5A1), which binds to the promoters of the steroid-producing genes, including steroidogenic acute regulatory protein (StAR) and aromatase ( Fig. 25.16 ). The presence of the transcription factor SF1 in endometriotic stromal cells is essential for the high levels of StAR and aromatase expression. Thus, the availability of a specific transcription factor (e.g., SF1) together with a receptor (e.g., EP2) determine expression (transcription) of the StAR and aromatase genes, whose promoters are not strongly regulated by epigenetic alterations.
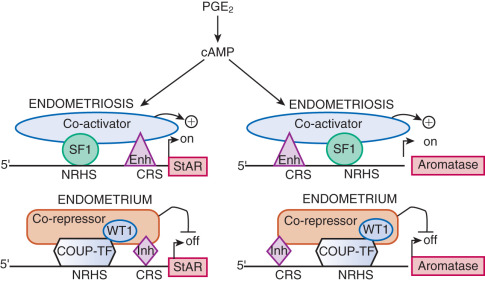
On the other hand, certain transcription factors (e.g., SF1, ERβ) are either present or absent in a cell type and their protein levels are ordinarily not induced by a specific stimulus. The expression of genes that encode them may be regulated by more robust mechanisms, such as DNA methylation of specific cytosine-(phosphate)-guanine (CpG) sequences ( Fig. 25.17 ). Under physiologic circumstances, a dense cluster of CpGs (an “island”) within the promoter region of the SF1 gene is not methylated and therefore not repressed in ovarian granulosa cells that contain abundant SF1 and its target aromatase, whereas the SF1 promoter is methylated and repressed in normal endometrial stromal cells that do not express SF1 or aromatase (see Fig. 25.17 ). The endometriotic stromal cell, however, manifests a partial characteristic of the ovarian granulosa cell and expresses SF1, which, under the stimulation of PGE 2 and cAMP production, binds to the promoters of key steroidogenic genes in a coordinated fashion. This effectively catalyzes the local conversion of cholesterol to progesterone and estradiol in a stepwise fashion in endometriosis.
Epigenetic regulation of gene expression and protein production is the hallmark of stem cell function and the differentiation of progenitor/stem cells to more mature cell populations giving rise to specialized organ function. The proposed key roles of endometrial tissue stem cells in the development of endometriosis (see above) underscore the indispensable function of epigenetic mechanisms in the pathogenesis of endometriosis.
DNA Methylation
Endometriotic cells express variable levels of the DNA methyltransferase enzymes (DNMTs), which introduce and maintain DNA methylation on the C5 position of cytosine in CpG dinucleotides. Several investigators have explored pathologic gene regulation in endometriosis related to differential DNA methylation between the eutopic endometrium and endometriosis, as well as between the eutopic tissues from women with or without endometriosis. Abnormal DNA methylation in endometriosis affects the expression of several genes, including homeobox A10 (HOXA10) , estrogen receptor-β (ERβ, ESR2 ), and SF1 (NR5A1) , which alter steroid signaling and responsiveness, and are critically involved in development and decidualization (see Fig. 25.17 ). In a murine model, normal endometrium placed in an ectopic location to create experimental endometriosis led to characteristic changes in gene expression in eutopic endometrium. These data suggest a possible effect of the inflammatory environment in lower abdomen that alters endometrial gene expression through altered epigenetic programming.
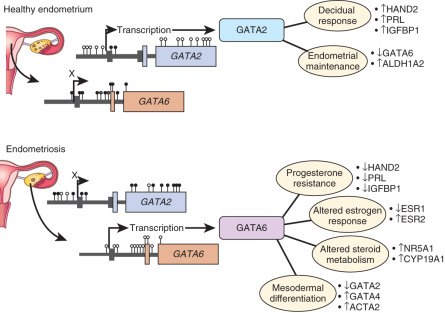
Genome-wide differences in DNA methylation between healthy human endometrial and endometriotic stromal cells have been mapped and correlated with gene expression using an interaction analysis strategy. Significant differences in methylation were mapped to some 400 genes, which included a disproportionally large number of transcription factors implicated in the pathology of endometriosis and decidualization. Differential methylation affected the HOX gene clusters, nuclear receptor genes, and intriguingly, the GATA family of transcription factors.
Functional analysis of the GATA family revealed that GATA2 regulates key genes necessary for the hormone-driven differentiation of healthy stromal cells, but is hypermethylated and repressed in endometriotic cells. GATA6 , which is hypomethylated and abundant in endometriotic cells, potently blocks hormone sensitivity, represses GATA2 , induces markers of endometriosis such as aromatase, SF1, and ERβ, and represses ERα and PR when expressed in healthy endometrial cells ( Fig. 25.18 ). The unique epigenetic fingerprint in endometriosis suggests that DNA methylation is an integral component of the disease, and identifies a novel role for the GATA family as key regulators of uterine physiology—aberrant DNA methylation in endometriotic cells correlates with a shift in GATA isoform expression that facilitates progesterone resistance and disease progression (see Fig. 25.18 ).
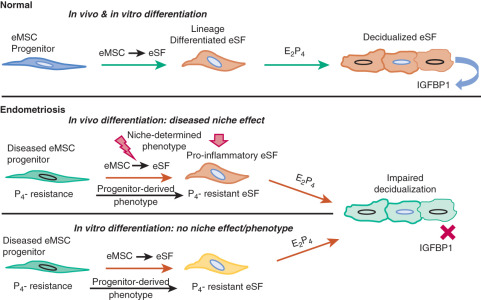
A recent study showed that, in contrast to endometrial mesenchymal stem cells from disease-free patients, eutopic endometrial mesenchymal stem cells isolated from endometriosis patients did not differentiate in vitro to stromal cells that decidualize properly. This suggested that the observed pro-inflammatory and progesterone-resistant gene expression signature in eutopic endometrial stromal tissue from endometriosis patients originates from defective endometrial mesenchymal stem cells ( Fig. 25.19 ). Together with the DNA methylation defects observed in eutopic endometrium of endometriosis patients, these findings substantiate the extraordinarily important and interactive roles of epigenetics and stem cells in the pathophysiology of endometriosis (see Fig. 25.19 ).
Nuclear Receptors and DNA Methylation
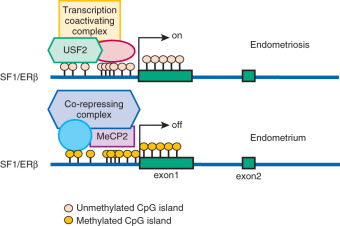
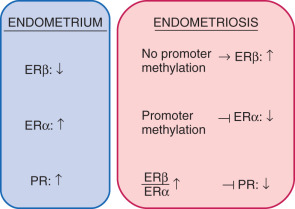
As described earlier, the levels of several nuclear receptors, including SF1, ERα, ERβ, and PR, are significantly different in endometriotic tissue compared with endometrium. The practical absence of the orphan (without a known ligand) nuclear receptor SF1 in endometrium and its 12,000-fold higher presence in endometriosis is, in part, determined by a classic CpG island at its promoter that is heavily methylated in endometrial stromal cells and unmethylated in endometriotic stromal cells (see Fig. 25.17 ). In endometrial cells, the silencer-type transcription factor methyl-CpG-binding domain protein 2 is recruited to the methylated SF1 promoter and prevents its interaction with transcriptional activators (see Fig. 25.17 ). In endometriotic cells, the transcription factor upstream stimulatory factor-2 (USF2) binds to the unmethylated SF1 promoter to activate it. In vivo, USF2 levels are strikingly higher in endometriosis compared with endometrium. Thus, differential SF1 expression in endometriosis versus endometrium is primarily controlled by an epigenetic mechanism that permits binding of activator versus inhibitor complexes to its promoter.
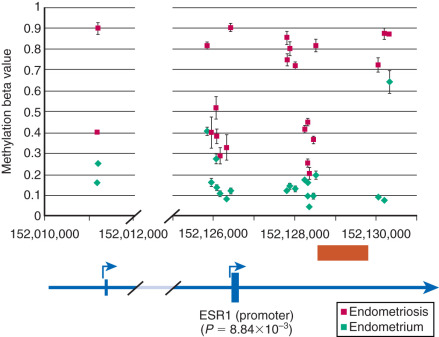
Among estrogen receptors, ERβ (ESR2) levels are 142-fold higher, whereas ERα levels are ninefold lower, in endometriotic tissue compared with endometrium (see Fig. 25.17 ). Hypomethylation of a CpG island at the promoter region of the ERβ gene allows high levels of expression in endometriotic stromal cells, and hypermethylation silences it in endometrial stromal cells (see Fig. 25.17 ). In contrast, the ERα (ESR1) promoter is appropriately unmethylated in eutopic endometrium and heavily methylated in endometriosis ( Fig. 25.20 ). Consequently, ERα levels are strikingly lower in endometriotic versus endometrial stromal cells. Therefore, it is conceivable that the pathologically high ERβ:ERα ratio in endometriotic stromal cells perturbs estradiol induction of the PR gene, giving rise to low PR expression in endometriosis ( Fig. 25.21 ).
MicroRNAs
MicroRNAs (miRNAs) are endogenous, small, highly conserved, noncoding RNAs which, in general, negatively control gene expression via targeting mRNAs, thus providing another layer of epigenetic regulation. In the eutopic endometrial tissues of women with or without endometriosis, global miRNA profiling showed dysregulation of the miR-9 and miR-34 miRNA families. HOXA10 is aberrantly regulated in the endometrium of women with endometriosis due to increased levels of both miR135a and miR135b, which target this gene. An altered microRNA profile in the endometrium may also suppress genes required for implantation. Another study revealed 10 microRNA species that are up-regulated (miR-202, 193a-3p, 29c, 708, 509-3-5p, 574-3p, 193a-5p, 485-3p, 100, and 720) and 12 microRNAs that are down-regulated (miR-504, 141, 429, 203, 10a, 200b, 873, 200c, 200a, 449b, 375, and 34c-5p) in ovarian endometriomas compared with endometrium. A recent study showed that aromatase inhibitor treatment significantly increases expression of let-7f in endometrial stromal cells from patients with endometriosis, whereas increased let-7f expression effectively reduces the migration of endometrial cells. These studies collectively suggest clinically relevant biological roles of miRNAs in the pathology of endometriosis.
Estrogen and Progesterone
Estrogen Formation and Metabolism
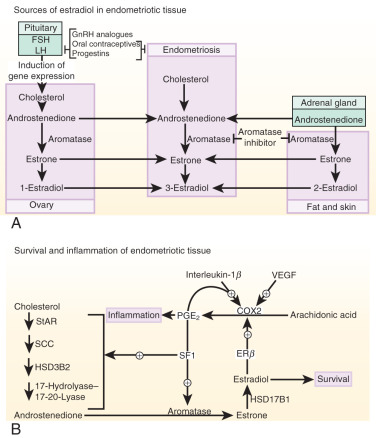
Estrogen production plays a key role in the pathology of endometriosis because its inhibition by gonadotropin-releasing hormone analogues (GnRHa), oral contraceptives, progestins, and aromatase inhibitors significantly reduces pelvic disease and pain ( Fig. 25.22A ). StAR facilitates the initial step of estrogen formation, the entry of cytosolic cholesterol into the mitochondrion. Then, six enzymes (side chain cleavage enzyme, 3β-hydroxysteroid dehydrogenase-2, 17-hydroxylase/17-20-lyase, aromatase, and 17β-hydroxysteroid dehydrogenase-1 [HSD17B1]) catalyze the conversion of cholesterol to biologically active estradiol ( Fig. 25.22B ). The key step , the conversion of C 19 steroids to estrogens, is catalyzed by aromatase, inhibition of which effectively eliminates all estrogen production ( Fig. 25.22B ).
Origins of Estrogen in Endometriosis
Three major body sites produce estrogen in women with endometriosis. First, estradiol secreted by the ovary reaches endometriotic tissue via the circulation. Moreover, follicular rupture at each ovulation causes spillage of extraordinarily large amounts of estradiol directly onto pelvic implants. Second, aromatase enzyme produced by peripheral adipose and skin tissue catalyzes the conversion of circulating androstenedione to estrone that may reach endometriotic tissue via the circulation and then is converted locally to estradiol. Finally, cholesterol can be converted to estradiol locally in endometriotic tissue, which expresses the complete set of steroidogenic genes, including aromatase. Peripheral and/or local aromatase activity in endometriosis may be particularly important because a combination of a GnRHa plus an aromatase inhibitor is significantly superior to GnRHa only for providing long-lasting pain relief in women with endometriosis (see Fig. 25.22A ).
Connection Between Estrogen and Inflammation
Cell survival and inflammation are responsible for chronic pelvic pain and infertility in endometriosis. Estrogen enhances the survival or persistence of endometriotic tissue, whereas prostaglandins and cytokines mediate pain, inflammation, and infertility. A link between inflammation and estrogen production in endometriosis involves a positive feedback cycle that favors overexpression of key steroidogenic genes, most notably aromatase, overexpression of COX2, and continuous local production of estradiol and PGE 2 in endometriotic tissue (see Fig. 25.22B ).
PGE 2 Stimulates Estradiol Formation
PGE 2 coordinately stimulates the expression of all necessary steroidogenic genes, enabling the endometrial stromal cell to synthesize estradiol from cholesterol de novo. Among the six steroidogenic genes, the regulation of StAR and aromatase has been characterized extensively (see Figs. 25.16 and 25.22B ).
Both endometriotic and endometrial stromal cells express similar levels of the PGE 2 receptor subtypes EP 1 , EP 2 , EP 3 , and EP 4 . Activation of the EP 2 receptor raises intracellular cAMP, which is responsible for mediating induction of StAR and aromatase expression by PGE 2 in endometriotic stromal cells. In PGE 2 -treated endometriotic cells, SF1 binds coordinately and assembles an enhancer transcriptional complex at the promoters of the StAR and aromatase genes to induce their expression ( Figs. 25.16 and 25.22B ).
The redundant presence of a number of transcriptional inhibitors of StAR and aromatase promoters in endometrial cells provides a fail-safe system for silencing these steroidogenic genes. These repressors are chicken ovalbumin upstream transcription factor (COUP-TF), Wilms tumor-1 (WT1), and CAAT/enhancer binding protein-β (C/EBPβ). Levels of WT1 and C/EBPβ are much higher in normal endometrium than in endometriosis. In the absence of SF1, a transcriptional complex comprising these repressors occupies the steroidogenic promoters and suppresses them in endometrial cells (see Fig. 25.16 ).
In summary, upon PGE 2 induction, coordinate recruitment of SF1 to the promoters of the essential steroidogenic genes is the key event driving local estradiol synthesis in endometriotic stromal cells (see Figs. 25.16 and 25.22B ). Because aromatase inhibition blocks all estradiol biosynthesis, it is a suitable therapeutic target among the steroidogenic enzymes. Aromatase inhibitors diminish or eradicate endometriotic implants and associated pain refractory to currently available treatments. 3123
Estrogen Action
ERβ appears to be the key mediator of estrogen action in endometriotic stromal cells. Compared with eutopic endometrial stromal cells, endometriotic stromal cells show severely suppressed ERα and strikingly higher ERβ levels. A hypomethylated ERβ promoter region results in remarkably elevated ERβ mRNA and protein expression relative to the normal endometrium. In addition, the eutopic endometrium of women with endometriosis has elevated ERβ expression compared with the endometrium of healthy women, suggesting that high levels of ERβ in the endometrium may predispose women to endometriosis. Further studies demonstrate that in endometriotic stromal cells, ERβ transcriptionally represses ERα, indicating that elevated ERβ confers a unique estrogen response mechanism that may contribute to disease progression.
Genome-wide analysis of ERβ binding and gene expression profiles in human endometriosis and endometrial tissues identified Ras-like, estrogen-regulated, growth inhibitor (RERG) and serum and glucocorticoid-regulated kinase (SGK1) as key ERβ targets. Estradiol potently induces RERG mRNA and protein levels in human endometriotic stromal cells, whereas PGE 2 phosphorylates RERG and enhances its nuclear translocation. RERG induces ribosome biogenesis and the proliferation of primary endometriotic cells ( Fig. 25.23 ). Estradiol/ERβ and PGE 2 signals integrate at RERG, leading to increased endometriotic cell proliferation; this pathway represents a novel candidate for therapeutic intervention (see Fig. 25.23 ). Estradiol/ERβ also stimulates SGK1 expression and enzyme activity, leading to increased human endometriotic cell survival (see Fig. 25.23 ).
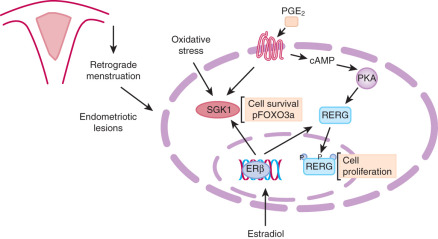
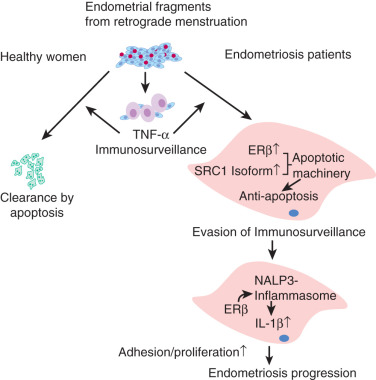
In a mouse model of endometriosis, with high ERβ levels, the inhibition of enhanced ERβ activity by an ERβ-selective estradiol antagonist suppressed mouse ectopic lesion growth, whereas gain of ERβ function stimulated the progression of endometriosis. As a mechanism to evade endogenous immune surveillance for cell survival, ERβ interacts with cellular apoptotic machinery in the cytoplasm to inhibit TNF-induced apoptosis. ERβ also interacts with components of the cytoplasmic inflammasome to increase IL-1β and thus enhance its cellular adhesion and proliferation properties.
Steroid Receptor Coregulators
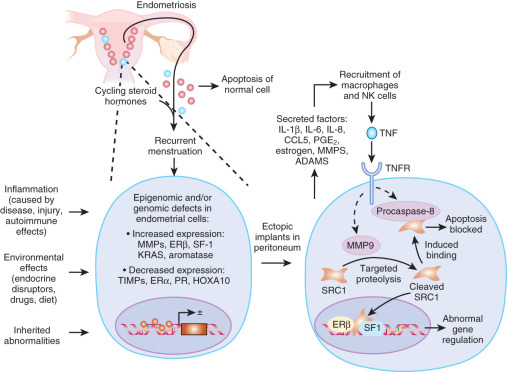
To precisely respond to external hormonal stimuli, steroid receptor coactivators (SRCs) dynamically modulate nuclear receptor-mediated cellular processes in a tissue-selective manner. Altered concentrations or modifications of SRCs in specific steroid-target tissues may be associated with nuclear receptor-dependent human disease progression. An SRC1-null mouse model reveals that the mouse SRC1 gene has an essential role in endometriosis progression. A 70-kDa SRC1 proteolytic isoform is highly elevated both in the endometriotic tissue of mice with surgically induced endometriosis and in endometriotic stromal cells biopsied from patients with endometriosis compared to normal endometrium. A TNF-induced matrix metallopeptidase 9 (MMP9) activity mediates formation of the 70-kDa SRC1 C-terminal isoform in endometriotic mouse tissue. In contrast to full-length SRC1, the endometriotic 70-kDa SRC1 C-terminal fragment prevents TNF-mediated apoptosis in human endometrial epithelial cells and causes the epithelial-mesenchymal transition and the invasion of human endometrial cells that are hallmarks of endometriosis. Thus, the TNF-MMP9-SRC1 isoform pathway promotes key pathologic functions in endometriotic tissue.
The nuclear actions of this recently described SRC-1 isoform are currently not known. It is possible that SRC1 or other nuclear receptor coregulators may modify the activities of key nuclear receptors, ERβ and SF1, in endometriotic stromal cells ( Fig. 25.24 ).
Progesterone Resistance
In contrast to a clear unfavorable effect of estrogen, the role of progesterone action in endometriosis has remained ambiguous for several reasons. First, the protective role of progesterone in endometrial cancer, characterized by epithelial proliferation, has been inappropriately attributed to endometriosis, which is benign and comprises primarily stromal cells displaying diminished apoptosis or differentiation. Paradoxically, progesterone induces a transient proliferation of stromal cells in normal endometrium during the secretory phase. Second, progestin-based treatments of pain are at best variably effective, especially in patients who previously received other forms of treatment. Progestins likely reduce pain via suppressing or attenuating ovulation. However, the direct effect of progestins on endometriotic tissue cannot be excluded. Third, a spectrum of antiprogestins with mixed agonist and antagonist properties (a.k.a., selective progesterone receptor modulators) reduces endometriosis-associated pelvic pain, possibly more effectively than progestins. To add a further twist, endometriotic tissue produces significant quantities of progesterone and contains strikingly lower levels of PR compared with endometrium. These seemingly disparate observations make it difficult to form a unified hypothesis regarding the role of progesterone in endometriosis.
PR Deficiency
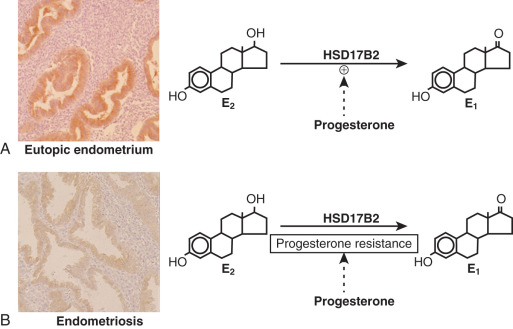
Progesterone resistance is readily explained by the extremely low PR levels in endometriosis (see Fig. 25.6 ). In the endometrium, levels of the PR isoforms, PR-B and PR-A, progressively increase during the proliferative phase, peak immediately before ovulation and diminish after ovulation, suggesting that estradiol stimulates PR levels. In contrast, PR-B is undetectable, and PR-A is markedly lower, in simultaneously collected tissues of endometriosis. Moreover, deficiency of the co-chaperone FKBP52, which is necessary for PR function in the endometrium of baboons with endometriosis, may contribute to progesterone resistance.
17β-Hydroxysteroid Dehydrogenase-2 Enzyme (HSD17B2) Deficiency
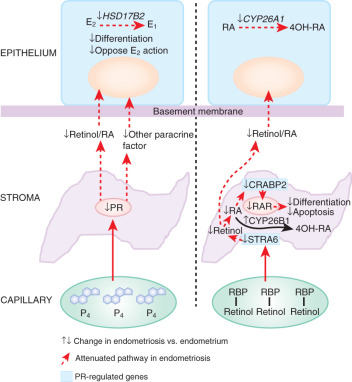
The first evidence for progesterone resistance in endometriosis was provided by the deficient expression of HSD17B2 in this tissue. In the endometrium, progesterone exerts its antiestrogenic effect in part via induction of HSD17B2, which catalyzes the conversion of biologically potent estradiol to much less potent estrone ( Fig. 25.25 ). Progesterone acts via PR in endometrial stromal cells to increase the formation of retinoic acid, which, in turn, induces HSD17B2 expression in endometrial epithelial cells in a paracrine fashion ( Fig. 25.26 ). However, endometriotic stromal cells fail to respond to progesterone and thus do not produce retinoic acid (RA), which is the direct physiologic stimulus for HSD17B2 induction (see Figs. 25.25 and 25.26 ). RA deficiency that is a consequence of progesterone resistance leads to the absence of epithelial HSD17B2 expression and the failure to inactivate estradiol in endometriosis (see Figs. 25.25 and 25.26 ). Combined with high estradiol production due to aberrant aromatase activity, this additional defect further contributes to the abnormally high levels of estradiol in endometriosis (see Figs. 25.25 and 25.26 ).
Retinoid Deficiency
STRA6 is a specific cell surface receptor for retinol binding protein (RBP) that binds retinol (vitamin A) and delivers it to other tissues via the circulating blood (see Fig. 25.26 ). Once retinol enters the cell, retinol and retinal dehydrogenase enzymes catalyze its conversion to transcriptionally active RA. RA serves as a ligand that regulates transcription through various RA receptors including (RAR)-α, -β, and -γ and peroxisome proliferator-activated receptor (PPAR)-β/δ (see Fig. 25.26 ). PR-deficient endometriotic stromal cells are unable to produce sufficient RA in part due to a deficiency of STRA6 that is strongly induced by a PR-dependent pathway (see Fig. 25.26 ).
Transcriptional activation of the nuclear receptor RAR often leads to inhibition of cell growth. However, in some tissues, RA promotes cell survival and hyperplasia, activities that are mediated by PPARβ/δ, which, in turn, induces the expression of prosurvival genes. The partitioning of RA between the two receptors is regulated by the intracellular lipid-binding proteins CRABP2 and FABP5. These proteins specifically deliver RA from the cytosol to nuclear RAR and PPARβ/δ, respectively, thereby selectively enhancing the transcriptional activity of their cognate receptors. Consequently, RA functions through RAR and is a proapoptotic agent in cells with a high CRABP2/FABP5 ratio, but it signals through PPARβ/δ and promotes survival in cells with a low CRABP2/FABP5 ratio. As for STRA6, PR is also a strong inducer of CRABP2 gene expression ( Fig. 25.26 ). This mechanism fits well into the overall model of PR deficiency in endometriotic stromal cells ( Figs. 25.6 and 25.26 ).
General Deficiency of Progesterone Action
Estrogen and progesterone are essential and sufficient to control the entire endometrial function via regulating the expression of hundreds to thousands of genes during the menstrual cycle. Indeed, administration of estradiol and progesterone is sufficient to prepare the endometrium for implantation in postmenopausal women undergoing donor embryo transfer. Progesterone exposure induces differentiation of both endometrial stromal cells (decidualization) and epithelial cells (secretory phenotype). Molecular markers of progesterone action include an increased production of epithelial glycodelin and stromal prolactin in endometrium. However, progesterone induces much lower levels of prolactin expression in endometriotic versus endometrial stromal cells, suggesting progesterone resistance in endometriosis. Gene expression profiling of the eutopic endometrium of women with endometriosis also exhibits progesterone resistance including glycodelin deficiency.
To summarize, progesterone resistance in endometriosis is associated with PR deficiency in poorly differentiated stromal cells, which gives rise to impaired paracrine signaling (e.g., RA) to epithelial cells (see Fig. 25.26 ). Compounds with mixed progesterone antagonist/agonist properties have effectively reduced uterine bleeding and pelvic pain associated with endometriosis. This may be due to their capacity to act via low levels of PR in endometriotic tissue and/or through a direct effect on the endometrium.
Inflammation
Inflammation is a hallmark of endometriotic tissue that overproduces prostaglandins, metalloproteinases, cytokines, and chemokines. Increased levels of acute inflammatory cytokines such as IL-1β, IL-6, and TNF likely enhance adhesion of shed endometrial tissue fragments on peritoneal surfaces, whereas proteolytic membrane metalloproteinases may further promote their implantation. MCP-1, IL-8, and RANTES attract granulocytes, natural killer (NK) cells and macrophages typically observed in endometriosis. Autoregulatory positive feedback loops ensure further accumulation of these immune cells, cytokines, and chemokines in established lesions.
Clear molecular distinctions have been observed between endometriotic tissue and endometrium in terms of the production of prostaglandins, cytokines, and chemokines (see Fig. 25.13 ). Intriguingly, more subtle forms of these abnormalities have been observed in eutopic endometrial tissues of endometriosis patients (see Fig. 25.13 ). In fact, gene expression profiling of endometrium from women with endometriosis versus that from disease-free women revealed a proinflammatory phenotype.
Immune System
The immune response to retrograde menstruation and pelvic endometriotic implants has been a key area of investigation in endometriosis. It is hypothesized that the local pelvic inflammatory process, with altered function of immune-related cells in the peritoneal environment, plays a crucial role in the pathogenesis of endometriosis. Some of the suggested immune system-related mechanisms include defective immunosurveillance, resistance of the endometrial cells to apoptosis and phagocytosis, and an associated decreased NK cell activity and cytotoxicity against the endometrial cells. Since immunologic factors are thought to be significantly involved in endometriosis, therapeutic manipulation of the immune system may play a beneficial role in its treatment. The potential immunomodulatory agents, including pentoxifylline, loxoribine, interferon-α 2b, IL-12, and TNF inhibitors were proposed as alternative approaches to the alleviation of pain and infertility. To date, however, none of these treatments have been proven to be of clinical benefit in a human trial.
Sources of Inflammatory Substances
Several studies suggest that the stromal cell in endometriosis tissue may be a key source of cytokines such as IL-6, IL-8, TNF, and RANTES. The endometrial or endometriotic tissue may also serve as a key source of prostaglandins; in this case, the epithelial cells seem to be the major source for prostaglandin formation. These data suggest that the endometriotic stromal cell may originate from a transformed endometrial stem cell population with an epigenetically acquired partial phenotype of an immune cell (e.g., a macrophage). The circulating immune cells accumulated in endometriotic implants are believed to be another major source of cytokines.
Cytokines and Ovarian Steroids
Estrogen and progesterone were reported to exert variable in vitro and in vivo effects on the production of different cytokines in endometrial or endometriotic cells. Recently an intriguing link was established between ERβ, TNF, and IL-1β in endometriotic tissue. This nongenomic action of ERβ may have a predominant role in endometriosis progression ( Fig. 25.27 ).