Introduction
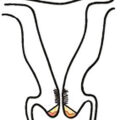
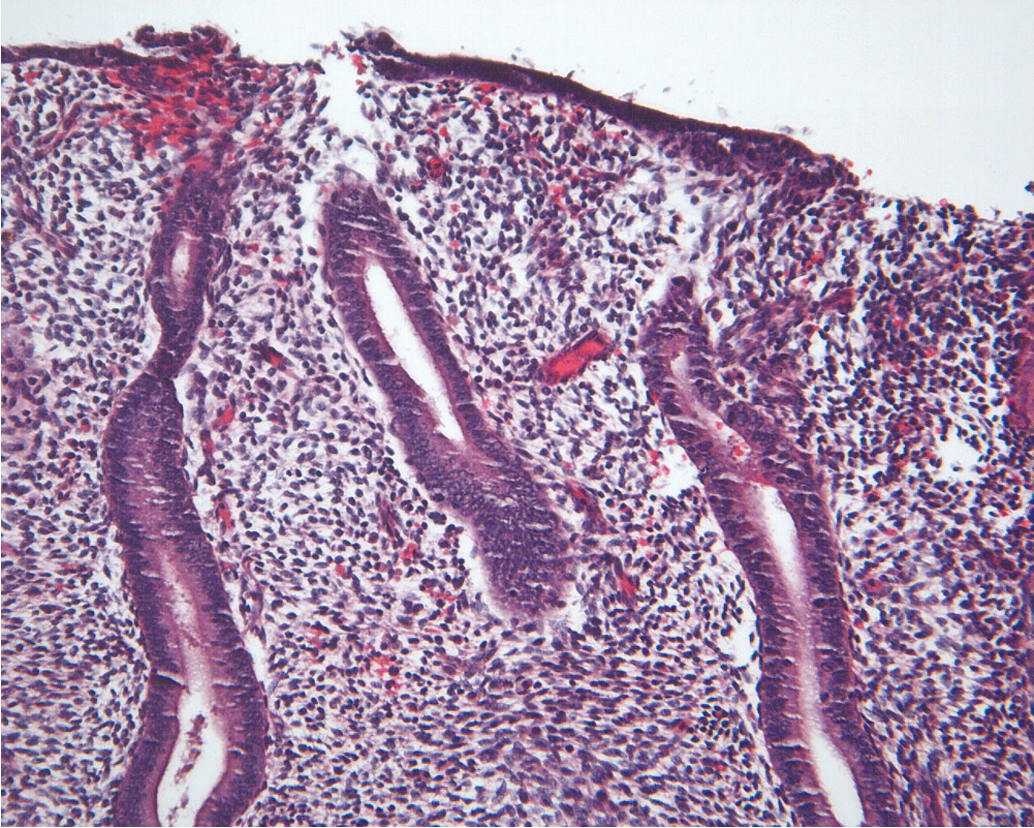
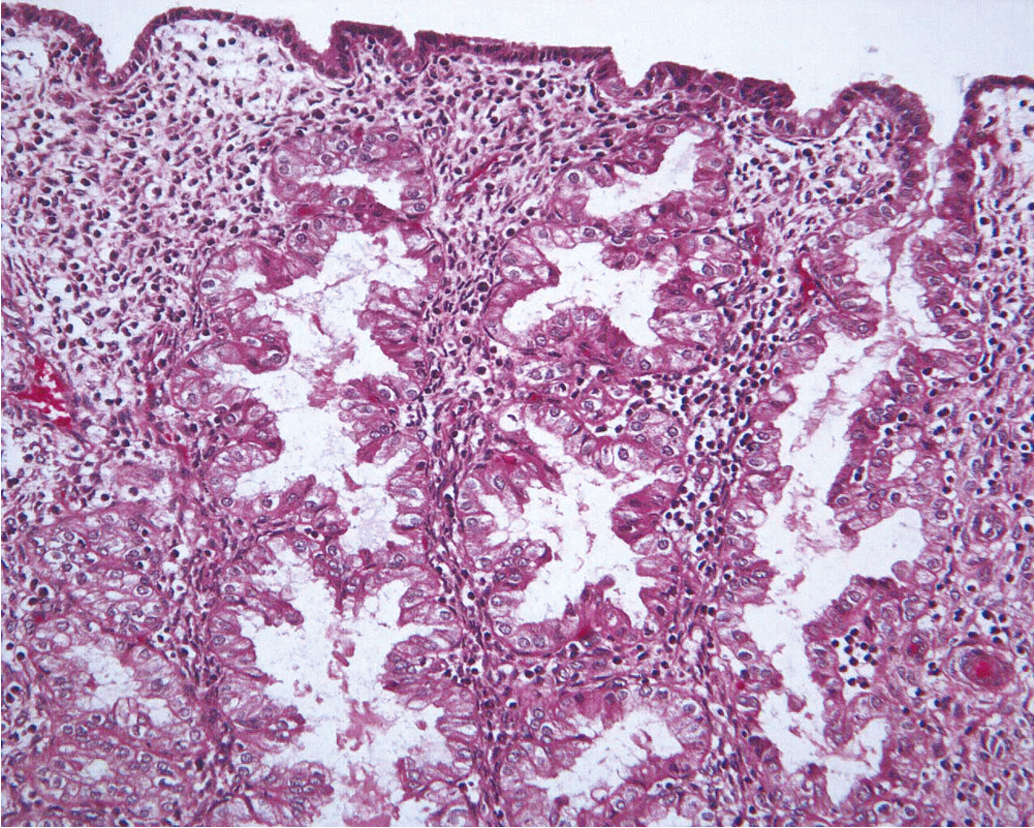
The endometrium is a dynamic tissue in reproductive age women. It is continuously cycling in response to hormonal, stromal, and vascular influences, with the ultimate goal of implanting an embryo and supporting the nutritional needs of the developing pregnancy. While estrogen stimulation is associated with the growth of proliferative endometrium ( Fig. 4.1 ), progesterone produced by the corpus luteum after ovulation inhibits proliferation and stimulates formation of secretory endometrium ( Fig. 4.2 ). Without conception and human chorionic gonadotropin (hCG) production, the corpus luteum fails to produce progesterone, and hormonal withdrawal allows endometrial breakdown and menses to occur. In contrast, continuous estrogen exposure bypasses the normal recycling of the endometrium and allows uninhibited endometrial growth, with the potential for neoplastic change.
Unopposed estrogen is not the only etiology of endometrial cancer, and it is important to note that a percentage of endometrial cancers will occur that are not hormonally driven. In fact, over the past decade, we have come to better understand the molecular foundations of endometrial cancer with four distinct molecular subtypes defined by The Cancer Genome Atlas (TCGA). This understanding has evolved from the traditional type I (estrogen excess) and type 2 (estrogen independent) dichotomy to a molecular classification that is more diagnostically reproducible, more prognostic, and more predictive of response to therapy.
The new classification has also changed the way in which we think about the etiology of endometrial cancer. Estrogen excess is the primary etiology of many, but not all cases of endometrial cancer. While traditionally we thought of estrogen excess in its many forms (obesity, polycystic ovary syndrome [PCOS], unopposed pharmaceutical estrogen compounds) as the primary cause of type I endometrial cancers, we now consider estrogen excess being most important in those tumors that are copy number low (low mutational burden) endometrial tumors. These are also the tumors that often present with or have concurrent preinvasive lesions and are most responsive to hormonal treatment. This new classification better defines these estrogen-related tumors with respect to prognosis as well as potential targeted treatments. Importantly, a certain percentage of estrogen-driven tumors will also be mismatch repair deficient (MMRd).
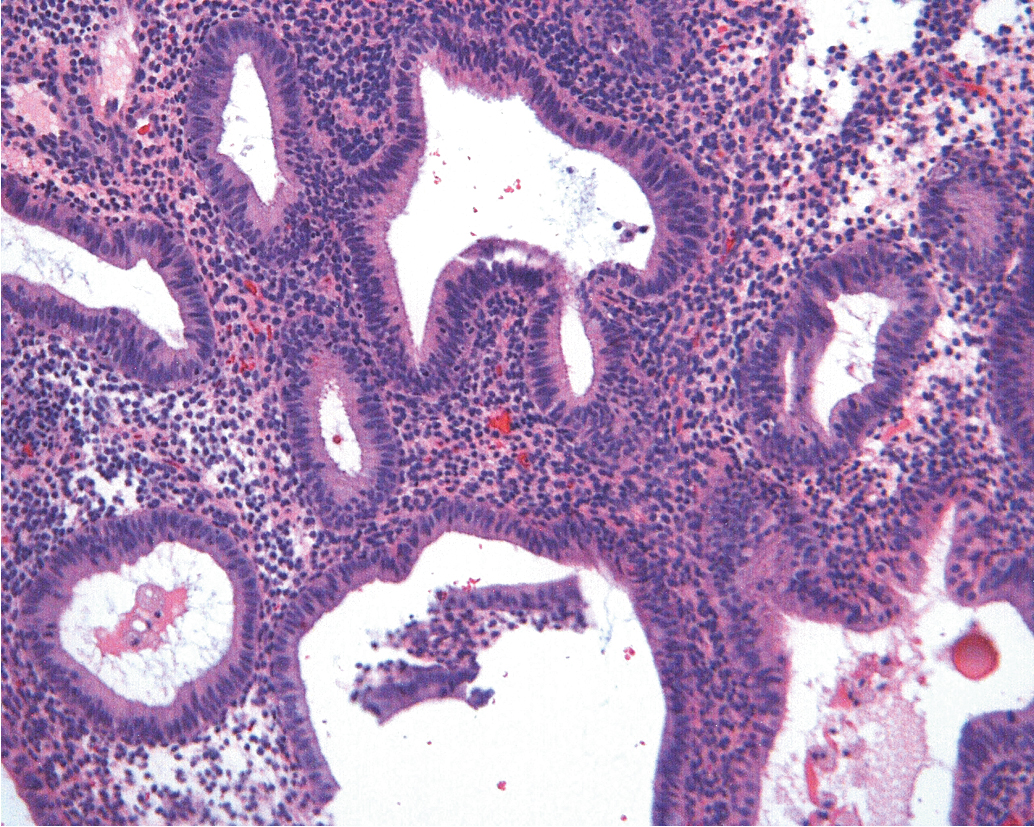
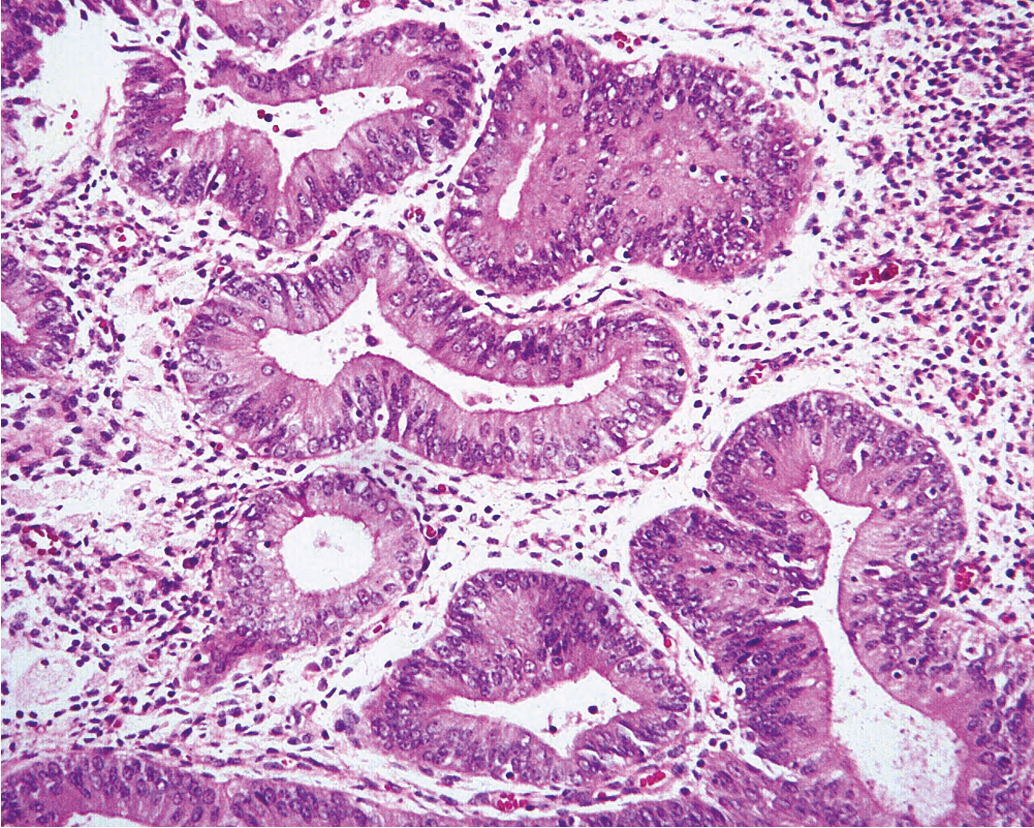
Historical data support the conclusion that unopposed estrogen stimulation yields a continuous spectrum of change from proliferative endometrium to benign endometrial hyperplasia ( Fig. 4.3 ) and subsequently through many variations of endometrial hyperplasia until a malignant neoplasm develops. Endometrial cancer is defined by the ability to invade local tissue and metastasize ( Fig. 4.4 ). Pre-malignant endometrial lesions may pre-date or co-exist with malignant endometrial cancer. Moreover, the histologic diagnosis of pre-malignant lesions can be made difficult by multiple factors including insufficient sampling or incorrect histologic interpretation. The development of new diagnostic subtypes (for example, endometrial intraepithelial neoplasia, or EIN) and novel diagnostic criteria have been helpful in better defining not only the diagnosis of pre-malignant lesions but also their propensity to progress to or be associated with a malignancy.
EIN is primarily caused by estrogen excess but responds to progesterone more frequently in premenopausal than in postmenopausal women. This dichotomy is multifactorial and deserving of further study. Progesterone therapy may also be effective initially but lose its effectiveness over time; research is needed to potentiate continued progesterone effectiveness in those women who either wish to preserve the corpus for fertility or are not reasonable candidates for definitive surgical therapy. The molecular classification of these lesions may assist us in a better understanding of which lesions will be most responsive to hormonal therapy alone, and which would benefit from the addition of other targeted agents.
Understanding the clinical situations in which excess unopposed estrogen may exist helps the clinician better predict which women are at risk for endometrial cancer and provides windows of opportunity for implementation of prevention strategies. The obesity epidemic has certainly contributed to the rising incidence of endometrial cancer in the US and other developed countries. Delays in childbearing in combination with a younger age of diagnosis has also led to more women with pre-malignant or malignant endometrial lesions wishing to preserve fertility.
Many female cancers contain estrogen receptors (ERs). The question of the role of hormone replacement therapy (estrogen with or without progestogen) in developing or stimulating cancers and its use for cancer survivors remains confusing, conflicting, and controversial. In the past, unopposed menopausal estrogen use contributed to the development of endometrial cancer. Today, we understand the importance of combining estrogen with a progestogen (progestin or progesterone) for women with an intact uterus. There are many Food and Drug Administration (FDA)-approved oral and transdermal therapies for hormone therapy (HT), that are available in varying doses. However, other modern trends are complicating the use of postmenopausal HT.
Treatment of gynecological cancers may have a significant impact on women and their quality of life. Early menopause (surgical or induced) has been shown in epidemiologic studies to have more health risk while acute onset of menopausal symptoms may be more debilitating than those seen with natural menopause. Understanding menopause and the benefits and risk of menopausal hormone use will help clinicians work with oncologists to respond to women’s menopausal concerns and improve quality of life for women at risk of or having survived cancer.
Data to provide definitive guidance regarding hormone replacement therapy is often lacking as the studies may be small or retrospective in nature. Menopausal symptoms of hot flashes, night sweats, and genitourinary syndrome of menopause may be more severe in women treated for cancer and the associated stress associated with diagnosis and treatment strategies may exacerbate the severity. Current literature suggests that it is important to include in decision making for an individual woman: what is known about the oncologic characteristics of the cancer, therapies used, grade and stage of the tumor including time of remission; information about the specific considered menopausal HT such as dose, formulation, use of estrogen alone or with a progestogen and duration of use; and lastly any endocrine characteristics such as hormone receptor status for the cancer, prior or future use of aromatase inhibitors, selective estrogen receptor modulators (SERMS), or other therapies . Due to a lack of large, randomized clinical trials showing safety of menopausal hormones in female cancers, specialists must rely on preclinical research, case reports, retrospective studies, any randomized controlled trials (RCTs), and meta-analyses to help predict the risks and benefits of menopausal HT for any given clinical scenarios. We will provide information about the known advantages and disadvantages for women with endometrial and breast cancer or whom are BRCA positive.
Endometrial hyperplasia: Pathologic diagnostic criteria
There are currently two systems of nomenclature for endometrial precursors, the 1994 World Health Organization (WHO94) schema and the EIN system. Recently, the American College of Obstetricians and Gynecologists and the Society of Gynecologic Oncology published their Committee Opinion endorsing the EIN system. The EIN system is both more reproducible and better associated with malignant potential. It also gives direction for therapeutic options. As pathologists and clinicians become more familiar with this nomenclature, most pathologists are reporting both the EIN diagnosis as well as the traditional hyperplasia categories. Since most historical data still utilize the hyperplasia nomenclature, we will discuss both nomenclatures below, and when discussing studies, will use the nomenclature utilized in the specific study. Additionally, it is critical to introduce the concept of EIC, endometrial intraepithelial carcinoma. This entity is very different from EIN and represents the precursor lesion for uterine serous endometrial cancer.
Traditional endometrial hyperplasia
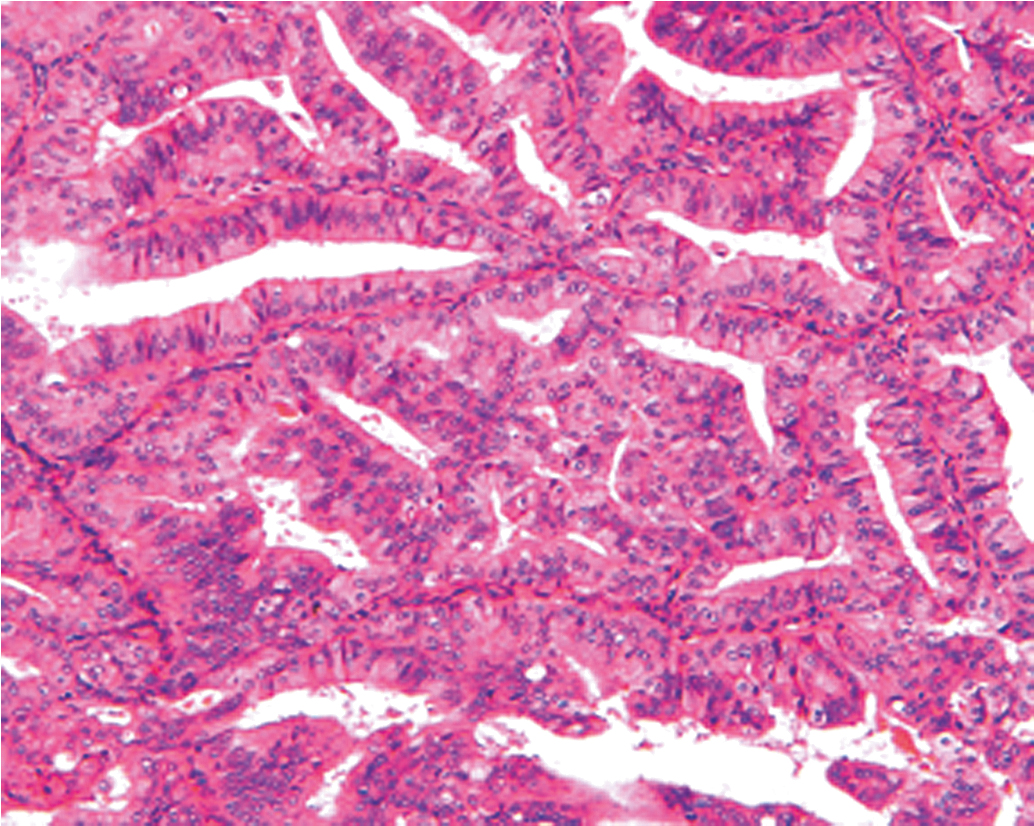
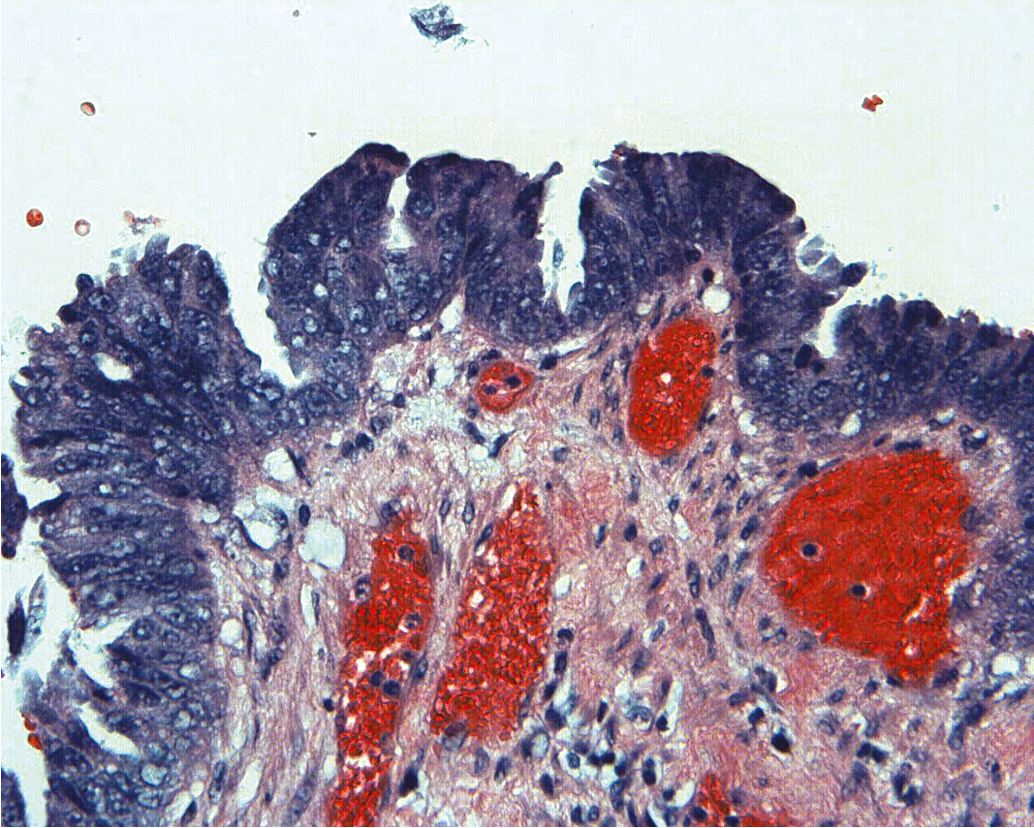
The traditional classification of endometrial hyperplasia divides hyperplasia into four categories based on architectural structure and cytologic features. The architecture is either simple or complex, and the cytologic features are described as with or without atypia. This yields four separate diagnoses ( Table 4.1 ): simple hyperplasia without atypia (see Fig. 4.3 ), complex hyperplasia without atypia, simple hyperplasia with atypia, and complex hyperplasia with atypia (AEH) ( Fig. 4.5 ). The terms adenomatous and cystic-glandular hyperplasia have been discarded, and when the abbreviation AEH is used, it refers to atypical endometrial hyperplasia (AEH), which reflects the two categories of hyperplasia with cytologic atypia (simple hyperplasia or complex hyperplasia with atypia). The presence of cytologic atypia is the most important criteria for progression to adenocarcinoma or the coexistence of endometrioid adenocarcinoma. The rates of coexisting endometrioid adenocarcinoma with AEH are reported to be as low as 13% and as high as 43%. Clinically, the term complex atypical hyperplasia (CAH) is used to describe hyperplasia that has both cytologic and architectural atypia and is the category most likely to progress (or be concurrent with) an invasive lesion.
|
Traditionally, the results of the retrospective “expert” review from Dr. Kurman’s group has been used to predict malignant capability of endometrial cancer precursors. From this classic study came the estimation that the risk of progression of CAH to endometrial cancer is 29%. Although this study added significantly to our understanding of the natural history of endometrial cancer precursors, it also had some significant limitations, including: the low number of patients with CAH (35), the retrospective nature, the fact that a year elapsed between the endometrial biopsy and the hysterectomy specimen, and the fact that only one pathology “expert” reviewed all the diagnoses.
Recognizing these limitations, a prospective study of AEH (both simple and complex) was undertaken by the Gynecologic Oncology Group (GOG). This study had two primary endpoints: to determine the reproducibility of the diagnosis when made in the “real-life” community setting, and to prospectively determine the concurrent/progression rate of AEH. Women with the community diagnosis of AEH who agreed to have a hysterectomy within 12 weeks of diagnosis of AEH were enrolled; the preoperative biopsy did not have to be reviewed by a GOG pathologist to allow study entry. Both the preoperative biopsy and the postoperative hysterectomy specimen were submitted to the GOG “expert” pathology panel for review. The study population included 302 eligible cases with a median age of 57 years; 31% were 50 years of age or younger.
The endometrial biopsy specimens were re-reviewed by three gynecologic pathologists independently (study panel diagnosis) in a blinded protocol. The “expert” reviewers agreed to categorize the endometrium as normal (cycling, menstrual, or atrophic), nonatypical hyperplasia (disordered proliferative, simple or complex hyperplasia), atypical hyperplasia (simple or complex), adenocarcinoma, or inadequate for evaluation. Two of three study pathologists had to agree on one of the above five diagnoses for a study panel diagnosis to be established. Correct diagnosis was determined by consensus agreement on the hysterectomy specimen during a simultaneous panel review with a multiheaded microscope. The results found only 40% of the cases had all three pathologists in agreement. The reproducibility was lowest for the diagnosis of AEH (kappa 0.28), and better for adenocarcinoma (kappa 0.51). Reproducibility was best for dilation and curettage (D&C) (kappa 0.47; confidence interval [CI] 0.41:0.53) compared with small sampling devices (kappa 0.26 to 0.36).
In addition to the lack of agreement among experts, there was a lack of agreement with the original diagnosis of the enrolling institution. Two of three study panel members agreed with the referring institution diagnosis of AEH in only 38% of cases. The study panel diagnosis was less severe in 25% of cases and more severe (adenocarcinoma) in 29% of cases. It is in large part because of the lack of reproducibility in the diagnosis of this pre-malignant lesion that the new nomenclature EIN is important.
Another important outcome of this study was the finding that 43% of the enrolled participants were found to have adenocarcinoma in their uterus at the time of hysterectomy. This number is obviously far higher than the 29% result from Dr. Kurman’s study. This number is arguably more accurate than 29%, given the prospective nature of the trial, the inclusion of multiple expert reviewers, and the required 12-week interval between biopsy and hysterectomy.
Perhaps most alarming, the uterine examination revealed the presence of some risk factors for metastatic disease present in 43 cases, including myometrial invasion or high-grade lesions. There were 123 patients that were determined to have endometrial cancer. Sixty-five percent (80/123) were confined to the endometrium; 77 of these cases had grade 1 disease and so were likely cured with hysterectomy alone. However, 31% had myoinvasive disease, and 10.6% had myoinvasion to the outer third. Eight of 123 endometrial cancer cases had endometrial cancers that were grades 2 or 3. These findings suggest a certain percentage of women with a preoperative diagnosis of AEH will have endometrial cancer with high risk factors at the time of hysterectomy. These women would have benefitted from surgical staging with a gynecologic oncologist to best treat their cancer and better inform the need for adjuvant therapy.
The findings of this critical study have changed the clinical management of women with AEH. First, given the higher-than-expected rate of concurrent endometrial cancer, most providers now recommend definitive hysterectomy for AEH provided the patient has completed childbearing and is a surgical candidate. Second, gynecologic oncologists either routinely perform sentinel node dissection on women with AEH or send frozen section with node dissection based on uterine factors. Third, women with AEH need to be carefully counseled with respect to ovarian preservation, given the high rate of concurrent endometrial cancer.
Endometrial intraepithelial neoplasia
In 2014, the World Health Organization (WHO) recognized that the goal of all classification schemas should be to bifurcate at clinical decision points. The new classification system of EIN attempts to simplify the previous hyperplasia designation into a binary system that aligns with clinical options and implications. The system is therefore divided into two categories: hyperplasia without atypia (indicative of hormonal milieu, and can be followed with hormonal therapy) and EIN. The EIN system developed by the International Endometrial Collaborative Group classifies endometrial precursors based on their clonal origin, noninvasive growth patterns, and risk of concurrent carcinoma. For a diagnosis of EIN to be made, the following criteria must be met: area of glands exceeds the stroma, cytology differs between the crowded focus and the normal background endometrium, the size of the lesion exceeds 1 mm, and benign pathology with overlapping features such as polyps or effects of exogenous estrogen can be eliminated.
There is evidence that the clinical outcome prediction and the interobserver reproducibility using EIN is improved over the 1994 WHO schema. EIN encompasses most of what was previously called CAH, but also includes cytologically bland proliferations which are morphologically distinct from the background endometrium (and would have been called SH or CH in the past). One study showed that EIN includes: 50% to 65% of what was previously called CAH, 45% to 60% of complex hyperplasia without atypia cases, and 5% to 10% of what was previously called simple hyperplasia.
One multicenter trial of 477 patients compared the WHO 1994 criteria to the EIN. When using WHO criteria, 13% of atypical hyperplasia (AH) progressed, and 2.3% of non-AH progressed. When using EIN, 19% of EIN progressed and 0.6% of non-EIN progressed. The sensitivity of EIN to detect a premalignant lesion was 92%, compared to 67% for the WHO criteria and 46% when using CAH alone. In a regression analysis, EIN was the strongest predictive index of future carcinoma.
The EIN diagnosis is more objective than the previous AEH diagnosis, allowing better reproducibility. The morphometric D score combines three quantitative variables in a mathematic formula; a score of less than 1 connotes a high rate of progression to endometrial cancer. The three variables include: stromal contribution, glandular complexity, and gland pleomorphism. The D score measurements were initially obtained objectively, but have been shown to correlate well with subjective interpretations. The EIN system also acknowledges the clonal presentation of endometrial cancer precursors. There are also molecular alterations associated with EIN. Loss of PTEN and PAX2 have been correlated with EIN lesions. Additionally, dissection and molecular testing have proven that PTEN/PAX2-negative areas represent distinct clones within the endometrium with malignant potential.
Endometrial intraepithelial carcinoma
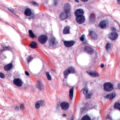
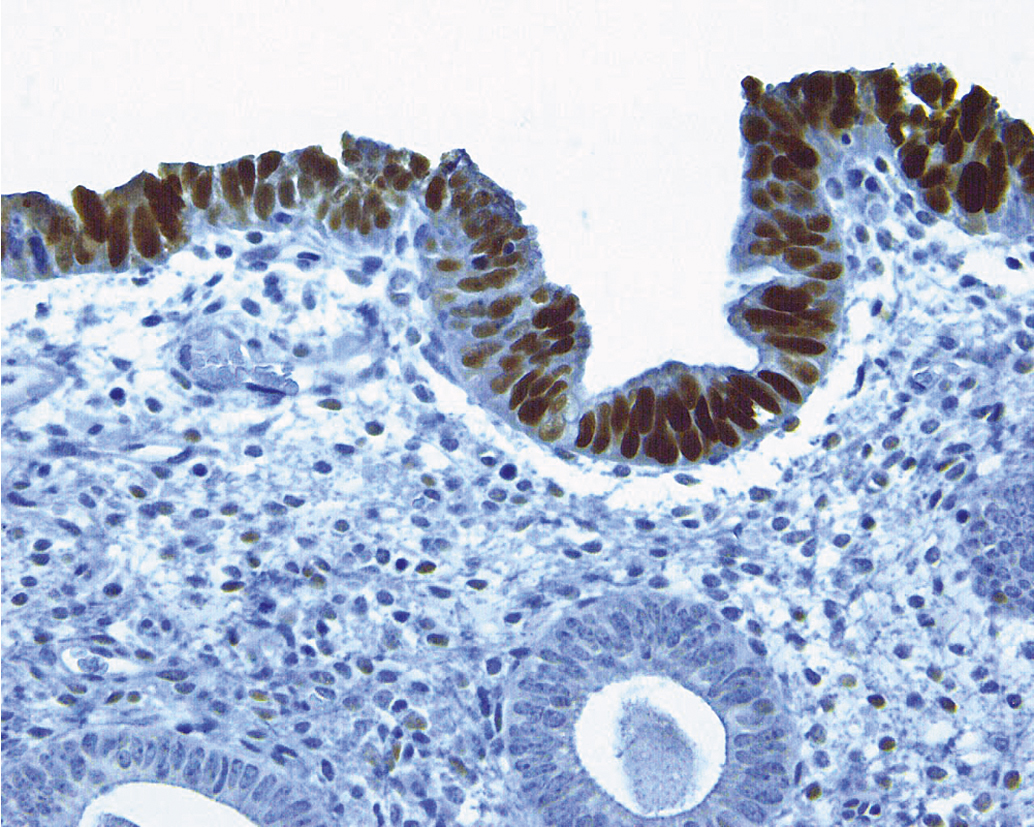
Serous carcinomas of the uterus arise in a background of atrophic endometrium. These cancers are not associated with excess estrogen and have been classified as type II (estrogen independent) endometrial cancers. Serous carcinoma is less likely to be identified at an early stage compared with endometrioid histologic types. These lesions are typically associated with mutations in the p53 tumor suppressor gene that results in accumulation of inactive p53 protein in the cell ( Fig. 4.6 ). Unlike normal cells or most endometrioid cancers, immunostaining with antibodies to p53 will be positive in the majority of serous cancers. Endometrial hyperplasia is not a precursor to serous carcinoma. Rather, the precursor has been identified as EIC. Serous EIC ( Fig. 4.7 ) can even be multifocal with disease found in the ovaries, fallopian tubes, and peritoneal cavity when no invasive component is found in the uterus. Comprehensive surgical staging is recommended because of the difficulty in establishing the diagnosis of invasive cancer intraoperatively. Postoperative therapy can be recommended after adequate histologic evaluation.
Clinical presentation
Women with EIN have a similar clinical presentation to those who have endometrial cancer with respect to symptoms. Pre- and perimenopausal women may present with irregular menses or oligomenorrhea consistent with a pattern of anovulatory cycles. Postmenopausal women may present with postmenopausal bleeding or an incidentally found thickened endometrial stripe. EIN may also present as atypical glandular cells on cytology in a woman who may or may not be symptomatic, or endometrial cells on cytology in a woman who is not menstruating at the time of cytology collection. Similar to endometrial cancer, investigation of new or unusual bleeding patterns or cytology findings as described previously with endometrial sampling is warranted.
There is evidence of exogenous or endogenous unopposed estrogen stimulation of the endometrium in most women with EIN, although EIN may be related to other factors . The most common causes of excess estrogen are obesity, polycystic ovarian disease, or a prolonged perimenopause with anovulatory bleeding patterns. Other rarer causes of estrogen excess include ovarian sources (such as an estrogen producing ovarian tumor) or adrenal causes. The classic presentation of a granulosa cell tumor of the ovary is a solid ovarian mass associated with abnormal uterine bleeding.
Approximately 4% to 6% of endometrial cancers may be due to an underlying hereditary cause, with Lynch syndrome accounting for the majority of these cases. Patients with Lynch syndrome have also been documented to have EIN alone and in association with cancer at the time of diagnosis. Previous studies suggest that Lynch syndrome–associated endometrial cancers appear to follow a similar stepwise continuum from EIN to cancer as that of sporadic cancers; however, the distinct molecular steps may be different for the two tumor types. Patients with Cowden syndrome carry a germline pathogenic variant in PTEN and are also at an increased lifetime risk for endometrial cancer. Given that PTEN somatic variants were the most common alteration seen in endometrial cancers in the Cancer Genome Atlas, it is no surprise that women born with a germline pathogenic variant in this gene are at an increased lifetime risk of endometrial cancer. Whether there is the same stepwise progression in these patients is largely unknown.
Office endometrial biopsy has replaced the D&C procedure for the diagnosis of endometrial hyperplasia or carcinoma; however, consideration of D&C is still recommended when considering conservative management to ensure accurate diagnosis. Historically, there was thought to be potential benefit of curettage as therapy, as well as providing more tissue for accurate histologic diagnosis. The use of “hormonal curettage” in which medical therapy with progesterone is followed by a withdrawal bleed provides the same benefit without the surgical cost or risk.
Management decisions for endometrial intraepithelial neoplasia
The patient’s age, desire for fertility, medical comorbidities, and personal preferences play a role in the management of these lesions. Total extrafascial hysterectomy with consideration of bilateral salpingo-oophorectomy (BSO) is generally recommended for women who are postmenopausal without a medical contraindication for surgery given that EIN is commonly found with coexisting cancer at the time of hysterectomy. Premenopausal women who have completed childbearing should also be counseled for hysterectomy, with or without BSO. As previously discussed, the GOG reported that 43% of 306 women who had a community-based diagnosis of AEH on endometrial biopsy and proceeded without medical treatment to hysterectomy had endometrial cancer on final pathology. Although 63% (77 of 123) of the cancers identified were grade I lesions confined to the endometrium, 31% were myoinvasive, and 11% had deep myometrial invasion into the outer 50% of the myometrium. It is possible that future validation of a new EIN scoring system or another molecular marker will allow more precise predictability for the neoplastic potential of EIN and the coexistence of cancer before hysterectomy. For the purpose of discussion of the available evidence, the nomenclature used in the particular trial will be discussed, recognizing the preferred classification now is EIN.
The complexity of the surgical management of EIN is underappreciated. The recommendation is that the patient should be informed of the increased risk of underlying malignancy and hysterectomy is required for the final diagnosis. The patient should also be counseled regarding ovarian retention or oophorectomy based on her age, family history, and other medical conditions or comorbidities. Intraoperative frozen section may be used to help guide the decision for oophorectomy; however, even with a diagnosis of invasive endometrioid adenocarcinoma of the uterus, the risk of adnexal metastasis is low at 5%. Genetic counseling and testing for Lynch syndrome may also be reasonable as a factor to consider in this decision-making process. For the vast majority of patients with EIN, lymphadenectomy and complete surgical staging results in overtreatment and increased surgical risk. Endometrial cancers associated with EIN are usually low-grade, early-stage lesions with a low risk of lymphatic metastasis.
Sentinel lymph node mapping has become standard of care in the surgical staging of endometrial cancers and previous retrospective studies have evaluated routine sentinel lymph node mapping in AEH. Touhami and colleagues reviewed 120 patients with AEH who underwent surgical staging with hysterectomy and sentinel lymph node mapping. Overall, 53% of patients were found to have endometrial cancer on final pathology and the majority of cases had stage IA disease. No patients with AEH had positive lymph nodes. The authors concluded that sentinel lymph node mapping can safely be omitted for patients with AEH only and no preoperative findings concerning for invasive carcinoma.
The risk of a high-risk uterine cancer in women with a biopsy of EIN ranges from 5% to 7%. In the event that high-grade cancer or deep myometrial invasion is found on final pathology that was not seen on frozen section or frozen section was omitted, reoperation may be offered for comprehensive surgical staging and removal of retained ovaries. Staging can usually be accomplished minimally invasively. This should be necessary in approximately 15% of the cases when cancer is identified and depends on the entire health history of the individual. Staging based on intraoperative assessment of myometrial invasion and grade of tumor is an appropriate strategy for institutions experienced in this approach. Supracervical hysterectomy, morcellation, and endometrial ablation are unacceptable for treatment of AEH because of the high prevalence of occult endometrial cancer in these patients.
Women who desire childbearing, refuse hysterectomy, or are medically inoperable can be treated hormonally with systemic progesterone therapy or a levonorgestrel-releasing intrauterine device. Although there are no evidence-based guidelines to outline ideal candidates for conservative management with hormonal therapy for EIN specifically, National Comprehensive Cancer Network (NCCN) does outline candidates for conservative management options for endometrial cancer (Version 1.2021), and these recommendations can be extrapolated for EIN. The diagnosis of EIN should be made on a D&C specimen to ensure adequate sampling of the endometrium and should be confirmed by expert pathology review. The American College of Obstetrician and Gynecologists (ACOG) Committee Opinion on Endometrial Intraepithelial Neoplasia (Number 631 May 2015) goes further to recommend consideration of hysteroscopy with D&C to include any discrete lesions as well as the background endometrium to ensure accurate diagnosis. For low-grade endometrial cancer, imaging with pelvic ultrasound or pelvic MRI is recommended to rule out myoinvasive disease. While there are not specific recommendations for EIN, transvaginal ultrasound is frequently performed during clinical evaluation of these patients.
In 2012, a systematic review identified 391 subjects from 45 studies with hyperplasia or grade 1 endometrial cancer who desired fertility and were treated with progestins. Seventy-eight percent of patients demonstrated a response to therapy with 53% of patients demonstrating a durable, complete response. The complete response rate was significantly higher for those with hyperplasia at 65% than for those women with carcinoma (48%).
Systemic progesterone therapy in the form of Megestrol 160 mg/day, in divided doses, has shown moderate success in the treatment of CAH with a response rate of 75% to 85%. Compliance with systemic progesterone therapy may be impaired by adverse effects of the treatment. Most commonly noted side effects that lead to decreased compliance include irregular vaginal bleeding, nausea, and weight gain, a particularly difficult treatment-related issue in an already obese patient population. Indeed, a recent qualitative analysis of cancer patients showed that almost half of patients reported nonadherence to oral anti-cancer regimens, including hormonal therapies. Side effect management emerged as one the key topics for non-adherence to oral medication.
Local therapy of the endometrium with a levonorgestrel-releasing intrauterine device has emerged as the preferred management strategy for most patients and has been evaluated in both the retrospective and prospective setting. In a multicenter randomized trial in Norway, 170 women with endometrial hyperplasia were randomized to one of three treatment arms: levonorgestrel intrauterine system (LNG-IUS), oral MPA 10 mg administered 10 days per cycle, or continuous oral MPA 10 mg/day for 6 months. At the end of 6 months, women in all treatment regimens showed significant therapeutic response, but the highest numbers of responders were noted in the LNG-IUS group (100%) and the continuous MPA group (96%) compared with the cyclical MPA group (69%). Gallos et al. reported a 12-year comparative cohort study of 344 patients with endometrial hyperplasia who received conservative management with progestins for complex or AEH because of desire for fertility or who were medically unsuitable for surgery. For the group of women treated with LNG-IUS ( n = 250), 94.8% achieved regression of hyperplasia compared with 84% of women treated with oral progestins. Of note, the patients with a body mass index of 35 or greater were most likely to have a relapse of hyperplasia or fail to regress to a normal endometrium. Pal and colleagues reported an 80% response rate with a levonorgestrel-releasing intrauterine device (IUD) in patients with CAH. In this cohort, body mass index (BMI) was not related to response to IUD; however, increased uterine size was a marker of non-response. Most recently, a phase II prospective trial of the levonorgestrel IUD in women with CAH and early-stage endometrial cancer reported a response rate of 90.6% in patients with CAH with a primary endpoint of pathologic response at 12 months. Importantly, quality of life was not significantly negatively affected. From a pathologic standpoint, the majority of responders had evidence of progesterone effect on biopsy by 3 months, while only 25% of non-responders demonstrated pathologic progesterone effect.
An important focus of future studies includes identifying markers for non-response to progesterone therapy in EIN and endometrial carcinoma, and several prospective studies are ongoing to evaluate whether the addition of other targeted therapies may improve response rates in this patient population. It is interesting to note that older (postmenopausal) women are less likely to respond to progesterone therapy. In one retrospective study of 41 postmenopausal women with AH and endometrial cancer who were treated with the levonorgestrel IUD, a complete response was noted in only 50%. Additionally, 4 of 18 patients with a complete response later experienced relapse of hyperplasia or cancer.
It is important to discuss with patients that frequent follow-up visits and resampling are needed with hormonal management to ensure response to therapy. There are no evidence-based guidelines for frequency and length of surveillance with hormonal therapy in EIN; however, there is consensus that regular sampling for at least the first year is needed in order to document response and follow-up schedule can again be extrapolated from NCCN guidelines for low-grade endometrial cancer (Version 1.2021). In general, response to hormonal therapy is seen by 6 months of treatment. Endometrial evaluation every 3 to 6 months with endometrial biopsy or D&C is recommended. If there is complete response by 6 to 12 months, conception is encouraged with continued progestin therapy recommended if the patient is not planning to actively try to conceive. In general, if hormonal therapy is utilized for fertility sparing indication, then completion hysterectomy, bilateral salpingectomy with or without oophorectomy should be considered after childbearing is complete. If there is no response at 6 to 12 months of hormonal therapy on pathologic review, hysterectomy should be considered for medically operable patients. Lifelong prevention strategies must be emphasized with patients and the diagnosis of EIN allows for important counseling on risk reduction.
In conclusion, management decisions in women with EIN are complex and require an understanding of the risk of invasive endometrial adenocarcinoma, the reproductive desires of the patient and her comorbidities, and the risks for surgical management. Ideally, minimally invasive techniques can be used to complete hysterectomy with BSO. The finding of adenocarcinoma in the uterus requires consultation with a gynecologic oncologist to determine whether observation, reoperation for surgical staging, or adjuvant therapy based on uterine factors should be considered.
Management of endometrial hyperplasia without atypia
The diagnosis of simple or complex hyperplasia without atypia, now collectively referred to as hyperplasia without atypia, requires hormonal management and is not an indication for hysterectomy. These lesions are generally reversible with progestogen (synthetic progestin or progesterone). The classification of progestogens is seen in Table 4.2 . Progestins may be given cyclically or continuously. Women, including teenagers, who are premenopausal and sexually active, are usually best treated with a regimen that provides contraception such as Depo-Provera or oral contraceptives. Women who want to conceive are likely to be anovulatory and require ovulation induction agents after withdrawal bleeding is produced with a progestogen. Reassurance can be obtained from a reduction of menstrual flow and a bleeding pattern that is appropriately timed with the cyclic therapy. It is extremely important to counsel anovulatory women about the lifelong risk of endometrial cancer and methods for surveillance and prevention. Twenty-five percent of endometrial cancers occur in the premenopausal age range, and 5% are in women 40 years of age and younger. These cancers may be part of the PCOS or the anovulatory transition before menopause, and are all theoretically preventable with proper counseling and active management. There is a dramatic rise in the incidence rate of endometrial hyperplasia and endometrial cancer at 45 years of age and peaking at 65 years of age. This is likely because of the combined effects of estrone (estrogen) production in the peripheral adipose tissue, particularly in obese women, and to the absence of progesterone that is caused by the loss of ovulatory function in menopause.
| Hormonal Agent | Dosage and Length |
|---|---|
| Medroxyprogesterone acetate | 10–20 mg/day or cyclic 12–14 days/month |
| Depot medroxyprogesterone | 150 mg intramuscularly every 3 months |
| Micronized vaginal progesterone | 100–200 mg/day or cyclic 12–14 days/month |
| Megestrol acetate | 40–200 mg/day |
| Levonorgestrel intrauterine system | 52 mg in steroid reservoir over 5 years |
Prevention of endometrial cancer
In premenopausal women, use of progestin containing contraception decreases risk for EIN and endometrial cancer, due to suppression of endometrial proliferation. Combined oral contraceptive pill (OCP) use is associated with a decreased lifetime risk of endometrial cancer with a 30% to 50% risk reduction in large epidemiologic studies. In addition, progestin-only contraception, including oral progestins, depot medroxyprogesterone acetate (depo-MPA), and progestin containing implants decrease incidence of endometrial cancer by as much as 80%. In Lynch syndrome, progestin use, with both depo-MPA and OCPs, has been shown to have tissue effect in a prospective intermediate biomarker study. In Lynch patients treated with 3 months of hormonal therapy, there was a decrease in endometrial epithelial proliferation and microscopic changes in the endometrium seen with progestin response.
In postmenopausal women, endometrial stimulation by estrogens unopposed by progestins leads to endometrial hyperplastic conditions in a dose- and time-dependent manner. Experimental evidence has been obtained from prospective clinical trials conducted to identify the appropriate doses and schedules of hormone replacement therapies. Kurman reported a randomized trial of 1176 postmenopausal women receiving 1 mg of estradiol orally and either placebo or various doses of norethindrone acetate. Women treated with estradiol alone (247 evaluable participants) for 12 months had a 12.2% rate of simple hyperplasia without atypia, 1.6% had complex hyperplasia without atypia, and 0.8% had CAH. Norethindrone acetate at all doses used in this trial nearly eliminated that risk. The North American Menopause Society supports the addition of a progestogen whenever estrogen is prescribed for menopausal symptoms in women with an intact uterus for the prevention of estrogen-induced hyperplasia and adenocarcinoma. Unfortunately, progestogen has undesirable side effects, and women have been known to discontinue this component of their prescribed hormone replacement (see Table 4.2 ).
The prevention of endometrial cancer has been complicated by the publication and early closure of the estrogen plus progestin (E + P) component of the Women’s Health Initiative (WHI). However, the WHI trial with conjugated equine estrogen (CEE) alone versus placebo had a very different outcome. There was a nonsignificant reduction in breast cancer risk ( P = .06), and the cardiovascular heart disease (CHD) risk was unaffected (hazards ratio [HR], 0.91; confidence interval [CI] 0.75 to 1.12), strokes were increased (HR, 1.39; CI 1.10 to 1.77), and hip fractures were reduced (HR, 0.61; CI 0.41 to 0.91). This places women with intact uteruses and their physicians in a difficult situation of balancing the risks. Unopposed estrogen use gives a woman a relative risk (RR) of endometrial cancer of three times the general population for less than 5 years of use and RR of 10 after 10 years of use. The risk decreases but remains elevated after discontinuing the drug. Alternatively, other progestins and progesterone formulations are available that may carry a different but as yet unknown risk of breast cancer. Micronized progesterone is available as oral 100- or 200-mg capsules (Prometrium) and has been approved by the FDA for use in combination with estrogen for relief of menopausal symptoms. The dosage of 200 mg for 12 days/month in a cyclic fashion causes withdrawal bleeding in a predictable manner. The regimen of CEE 0.625 mg/day with oral micronized progesterone at a dose of 200 mg/day for 12 days/month failed to see any increase in endometrial hyperplasia with 3 years of follow-up. A vaginal progesterone gel is available that is only FDA approved for infertility use but has been studied in small numbers of menopausal women. An additional alternative is to use the LNG-IUS (Mirena is FDA-approved for contraceptive use only) and unopposed estrogen, which has been studied prospectively in menopausal women. These women were only followed for 1 year, and they were able to convert proliferative endometrium to atrophic endometrium while using continuous estradiol 50 mcg/day via the transdermal route. The official position statement of the North American Menopause Society is that a progestogen should be added to estrogen therapy for all postmenopausal women with intact uteruses. The type, route, or regimen can be individualized to minimize side effects while providing adequate endometrial protection. Further discussion of hormone replacement therapy is discussed at length later.
For both premenopausal and postmenopausal women, obesity remains one of the most important and modifiable risk factors for EIN and endometrial cancer. Indeed, women with class III–IV obesity with a BMI of at least 40 kg/m 2 have a tenfold increased lifetime risk of endometrial cancer with a known increase in obesity-related premenopausal endometrial cancer cases in recent years. Lifestyle counseling and interventions are an important part of endometrial cancer prevention in this patient population; however, lifestyle changes remain one of the most difficult interventions to employ. A prospective randomized biomarker study of metformin and lifestyle intervention for the prevention of endometrial cancer in obese women demonstrated this, as 576 women were approached and only 52 patients attended the initial screening and 26 patients completed the study. This prospective evaluation showed significant decrease in weight with lifestyle intervention, but not metformin alone. In addition, there was no significant change in endometrial proliferation with either intervention, but overall initial proliferation rates were low.
Bariatric surgery may be indicated for some patients and prospective evaluation shows that weight loss associated with bariatric surgery led to resolution of atypical hyperplasia both with and without progestin therapy in patients who had abnormal endometrial pathology prior to bariatric surgery. MacKintosh and colleagues prospectively evaluated patients with endometrial biopsy prior to and following gastric bypass. Six of 72 women enrolled had atypical hyperplasia at enrollment and prior to bariatric surgery. Five patients had resolution of atypical hyperplasia at 12 months following surgery. In addition, weight loss with bariatric surgery was associated with a molecular response in the endometrium with decrease in endometrial proliferation, oncogenic signaling, and hormone receptor expression. Lastly, there were decreases in circulating biomarkers of insulin resistance and inflammation.
It is clear that obesity and excess circulating estrogen increases the risk for EIN and endometrial cancer; however, the interplay with other molecular factors are still largely unknown. Further studies are needed to better understand the molecular markers for potential risk of progression to EIN and cancer in obesity in order to better address prevention strategies moving forward. In addition, a collaborative, multi-specialty approach is needed in lifestyle interventions including dieticians, psychotherapy, and bariatric surgery for improved engagement and success for lifestyle interventions.
Benefits and risks of menopausal hormone therapy
Menopausal HT remains the gold standard for relief of menopausal symptoms (hot flashes, night sweats, and sleep disruption); reduction of bone loss and osteoporotic fracture risk; and, when used locally, relief of genitourinary syndrome of menopause (vaginal atrophy, urinary symptoms). Menopausal symptoms can be long lasting. As an example, vasomotor symptoms were shown in a longitudinal US study (SWAN) to last a median of 7.4 years, with ethnic variation from 5 years for Asians, 7 years for Caucasians, 9 years for Hispanics, and 10 years for African American women.
HT is felt to have the most benefits in symptomatic healthy women initiating HT when under age 60 or within 10 years of menopause onset. In general, initiating systemic HT in women who are older than 60 years is not recommended. HT is generally recommended for 3 to 5 years or to consider discontinuation by around the age of 60.
Risks of HT appear to vary depending on type, dose, duration, route of administration; the timing of initiation, and whether a progestogen is needed. Progestogens protect against endometrial hyperplasia and cancer that can occur from chronic unopposed exposure to estrogens. Estrogen therapy used alone is recommended for women who have had a hysterectomy, whereas combined estrogen-progestogen therapy is needed for women with an intact uterus. When combined with estrogen therapy, progestogens are given either sequentially or continuously. Sequential treatment typically results in withdrawal bleeding similar to a menstrual period, whereas continuous progestogen therapy eventually leads to cessation of bleeding.
Women with cancer, and those who are at risk for cancer, require a complex evaluation of the risks and benefits of hormonal therapy at the time of natural or induced (surgically or medically) menopause. These women may also consider alternatives to hormones for symptom management and prevention of osteoporosis and heart disease. The data describing risks, benefits, and alternatives for all menopausal women in general and women with or at risk for gynecologic or breast cancers are discussed in the following sections.
Hot flashes
Although the underlying physiology leading to vasomotor symptoms (hot flashes, night sweats, sleep disturbances) remains incompletely understood, abnormal functioning occurs in the thermoregulatory nucleus of the hypothalamus which regulates perspiration and vasodilatation. Core body temperature is normally maintained in a regulated range, called the thermoregulatory zone. It is hypothesized that women who experience more severe vasomotor symptoms have a narrower thermoregulatory zone than those without symptoms; minimal changes in core body temperature may lead to hot flashes or cold chills. Various hormones and neurotransmitters modulate hot flush frequency. Of these, estrogens play a vital role.
Hot flashes are characterized by a sudden increase of blood flow, often to the face, neck, and chest that may last 1 to 5 minutes, associated with the sensation of extreme heat or sweating, and may cause significant sleep disruption leading to fatigue and mood disturbances. Although approximately 75% of women have hot flashes, only 25% are bothersome enough for women to seek treatment. HT remains the most effective treatment for hot flashes. In a meta-analysis of 24 randomized controlled trials with over 3000 women combined, a 75% reduction in hot flashes was found with estrogen alone or combined with progestin compared to placebo. The use of non-hormonal treatments for hot flashes and vaginal dryness is discussed in depth later.
Critical assessment of risk/benefit ratio of hormone therapy: The women’s health initiative data
Early observational studies suggested protection of cardiovascular disease (CVD) and dementia with the use of HT. However, in 2002, the first findings from the large randomized controlled trial, the WHI, found hormone replacement increased the risk of CVD, blood clots, stroke, and breast cancer, with more dementia found in the trial of women over age 65. Both the estrogen/progestin trial and the estrogen alone trials were stopped early to prevent possible harm. The combined therapy trial (estrogen-progestin therapy EPT], CEE with 2.5 mg of medroxyprogesterone acetate) was stopped at a median of 5.6 years when excess risks were found annually of coronary heart disease events of 0.6/1000 women, stroke 0.9/1000 women, and breast cancer 0.9/1000 women. The estrogen alone trial (ET, CEE 0.625 mg) for women without a uterus was stopped at median 7.2 years due to increased risk of stroke annually of 1.2/1000 women over placebo with no cardiovascular benefit seen. Subsequent post hoc analyses based on age and time from menopause onset found a higher risk of CHD and stroke in women who initiated HT after age 60, with more risk if starting after age 70. No increased risk of CHD or stroke was seen for those starting under age 60 or within 10 years of menopause onset, with a nonsignificant decrease in CHD for the estrogen alone group.
To explore the timing of HT initiation among WHI participants, Rossouw and colleagues looked specifically at the effect of HT on stroke and CHD rates across categories of age and years since menopause in the combined (EPT plus ET) trial. Women who initiated HT closer to menopause tended to have reduced CHD risk compared with the increase in CHD risk among women more distant from menopause. In evaluating data by age, for the age group of 50 to 59 years, the hazard ratio for CHD was 0.93, or two fewer events per 10,000 person years; for the age group 60 to 69 years, 0.98 or 1 fewer event per 10,000 person years; and for those 70 to 79 years, 1.26 or 19 extra events per 10,000 person years. HT increased the stroke risk—the hazard ratio was 1.32—and this risk did not vary significantly by age or time since menopause. Evidence is insufficient to determine that either ET or EPT should be initiated or continued for primary or secondary CHD prevention.
The 2013 ACOG committee report, reaffirmed in 2016, recommended that HT should not be used for the primary or secondary prevention of CHD, but rather women should be prescribed estrogen or estrogen plus a progestogen (when a uterus is present) at the onset of moderate or severe menopausal symptoms. The dose should be the lowest necessary to achieve the symptom control desired, and the duration of therapy should be 5 years or less. However, they recommended against routine discontinuation at age 65; instead, individual consideration of risks and benefits.
The risks of venous thromboembolic disease (VTE) are doubled when oral estrogen is used, with less risk if transdermal therapy is used and/or if lower doses are prescribed. Fewer cases of type 2 diabetes were seen in the treated groups in the WHI. An increased risk of dementia was seen in women in the WHI who were age 65 or older. Post hoc analysis with long-term follow up did not show any increased risk of loss of cognition in women who started therapy between the ages of 50 to 59. In the randomized controlled ELITE trial (Early versus late postmenopausal treatment with estradiol) of 643 postmenopausal women who received either 1 mg estradiol plus 45 mg progesterone gel sequentially for 10 days each cycle with uterus or placebo, oral estradiol therapy showed less progression of subclinical atherosclerosis (measured as carotid artery intima media thickness or CIMT) than those on placebo when therapy was initiated within 6 years after menopause but not when it was initiated 10 or more years after menopause. These findings support the “timing hypothesis” of cardiovascular benefit and a possible cognitive benefit if HT is started closer to menopause onset (within 10 years) or when women are less than age 60. The lower absolute risks for younger women suggest that younger symptomatic women are most likely to have benefits exceed risks for menopausal HT.
Cumulative 18-year follow up of WHI participants (off therapy) found that all-cause mortality, cardiovascular, and total cancer mortality were similar between the HT and placebo groups . Breast cancer mortality at 18 years was elevated (HR 1.44) in the CEE/MPA group, which was statistically a nonsignificant increase, while a significant reduction in breast cancer mortality (HR 0.55) was noted in the CEE-alone group. A significant reduction (HR 0.85) in mortality from Alzheimer disease or dementia was seen with use of HT.
Cardiovascular disease and stroke
CVD risk factors for women include nonmodifiable risk factors such as age and family history of CVD. Modifiable risk factors include hypertension, dyslipidemia, obesity, diabetes/glucose intolerance, smoking, poor diet, and lack of physical activity. Vasomotor symptoms have been linked to indices of subclinical CVD; however it is not clear if vasomotor symptoms are markers for CVD or have a causative effect on CVD. Early menopause before the age of 50 has been shown to increase the risk of CVD with those under age 40 found to be twice as likely to have a cardiovascular event before the age of 60. Interpersonal violence, such as survivors of intimate partner violence, sexual abuse or assault, or human trafficking, has an association with increased risk of heart disease and should be considered when identifying risk factors for CVD.
Cardiovascular risk reduction recommendations include smoking cessation, exercise, and weight control along with blood pressure (BP) and cholesterol management. A healthy diet should be recommended, preferably plant-based or Mediterranean-like diet, high in vegetables, fruits, nuts, whole grains, lean vegetable or animal protein (preferably fish), and vegetable fiber. Central fat distribution, i.e., truncal obesity, correlates positively with increases in total cholesterol, triglyceride, and low-density lipoprotein (LDL) cholesterol levels, and negatively correlates with HDL (protective) levels. More than 5% weight loss has been shown to improve BP, LDL-C, triglycerides, and glucose levels among obese or overweight individuals, and to delay the development of type 2 diabetes. Physical activity of 150 minutes/week of moderate intensity or 75 minutes/week of vigorous intensity is recommended, along with strength training.
After menopause as estrogen levels decline, HDL levels decrease and total cholesterol levels increase, leading to a doubling of the risk of CHD for women by age 60. Dietary modification and statin therapy (when needed) can reduce LDL levels leading to a reduction in CVD. Women who smoke should be strongly advised to quit with consideration of behavioral modification, nicotine replacement, or other drug treatments.
Aspirin is not recommended for primary prevention of heart disease in women less than 65 unless health benefits are judged to outweigh risks. Aspirin lowered the risk of stroke by 17% (RR, 0.83; CI 0.69 to 0.99; P = .04) in a RCT of 39,876 healthy women aged 45 or older who received 100 mg or aspirin or placebo on alternate days for 10 years. Benefit in prevention of myocardial infarction was only found in women aged 65 years of age and older at enrollment (RR, 0.66; CI 0.44; 0.97; P = .04). The American College of Cardiology 2019 recommendations suggest considering low-dose aspirin for primary prevention only for higher-risk women ages 40 to 70 years who are not at increased bleeding risk and not to start or use it routinely for primary prevention or for those aged over 70 years or if there is an increased risk of bleeding regardless of age.
Cardiovascular disease and cancer risk
Cardiotoxicity can develop during or after cancer treatment. Heart damage caused by chemotherapy-induced cardiotoxicity has been shown to reduce quality of life and increase the risk of death from cardiac-related causes. Potential causes include chemotherapy, radiation therapy to the chest due to the proximity of the heart and lung to the radiation field, molecular therapy, monoclonal antibodies, and drugs used to prevent cancer recurrence.
Congestive heart failure is the most serious cardiotoxicity related to cancer treatment, while pericarditis (inflammation of the heart muscle) and coronary artery disease can occur. Less commonly, changes in BP (low or high), or arrhythmias or valvular disease can occur. Women who have additional cardiovascular risk factors appear to be at highest risk including those with history of smoking, obesity, high fat diet, sedentary or inactive lifestyle, or family history of heart disease.
The largest study used the Surveillance, Epidemiology and End Results (SEER) database looking at deaths from CVD (including CHD, hypertension, cerebrovascular disease, blocked arteries, and damage to the aorta). Analyses were adjusted for age, race and sex, looking at 28 different types of cancer. Among the 3,234,256 cancer patients, 38% (1,228,328) died from cancer and 11% (365,689) died from CVD—76% due to heart disease. The risk of dying from CVD was highest in the first year after a cancer diagnosis and for patients younger than 35 years. The majority of CVD deaths occurred in patients with cancers of the breast (a total of 60,409 patients) and prostate (84,534 patients), as these are among the most commonly diagnosed cancers. In 2012, 61% of all cancer patients in the study who died from CVD were diagnosed with breast, prostate, or bladder cancer. The proportion of cancer survivors dying from CVD was highest in bladder (19% of patients), larynx (17%), prostate (17%), womb (16%), bowel (14%), and breast (12%). Those who died were more likely to have the most aggressive and hard-to-treat cancers including cancers of the lung, liver, brain, stomach, gallbladder, pancreas, esophagus, ovary, and multiple myeloma. Thus cancer survivors from breast, larynx, skin, Hodgkin lymphoma, thyroid, testis, prostate, endometrium, bladder, vulva, and penis were about as likely to die of cardiovascular diseases as to die of their initial cancer.
A second study, the Framingham Heart Study, evaluated data over 15 years from 12,712 participants (average age 51) without CVD or cancer at study initiation. The American Heart Association/American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator and biomarkers were used to measure cardiovascular risk. During the study 1670 cancer cases were identified (19% gastrointestinal; 18% breast; 16% prostate; 11% lung). Increased risk of CVD factors of age, sex, hypertension, and smoking were independently associated with cancer. Those with a 10-year ASCVD risk of 20% or higher were more than three times as likely as those with 10-year ASCVD risk of 5% or lower to develop any type of cancer. Those who developed CVD (myocardial infarction, congestive heart failure, or atrial fibrillation) had a 7-fold increased risk for subsequent cancer while those with elevated brain natriuretic peptide (BNP) were more likely to get cancer than participants with low levels of BNP. Increasing awareness of these risks will hopefully lead to healthier lifestyle behaviors by cancer survivors and increased awareness by their providers about their increased risk of CVD, particularly if cancer survivors have underlying risk factors for CVD.
Osteoporosis
Osteoporosis is a disease characterized by low bone mass, microarchitectural deterioration of bone tissue, and a decline in bone quality leading to increased bone fragility and risk of fractures, particularly of the spine, hip, shoulder, and wrist. Peak bone mass occurs before the age of 30, then begins to decrease around age 45. Menopause induces an accelerated bone loss, followed by a slower rate of bone loss. Within the first 5 years of menopause, women can lose up to 20% of bone mineral density (BMD) and up to 30% by age 80. Risk factors for osteoporosis ( Table 4.3 ) include low body weight, history of eating disorder or prior gastric bypass, advancing age, either parent with prior hip fracture, history of smoking or excess alcohol, some long-term medications such as corticosteroids or aromatase inhibitors, and medical problems such as rheumatoid arthritis.