Introduction
Hormones flow from the ductless glands into the circulation and regulate the metabolism of the body, and with ageing there is a decline in the circulating levels of a number of hormones. Deficiency of some of these hormones produces symptoms and signs similar to the changes seen with ageing. This has led different authorities to suggest that ageing is due to an endocrinopause and that replacement of one or more hormones will result in a reversal of the ageing process. Thus, it has been claimed that the ageing process is due to the somatopause, adrenopause, menopause or andropause. However, hormonal replacement has been as likely to produce negative effects as it has to lead to rejuvenation. Ageing is also associated with changes at the receptor or postreceptor level that can alter hormonal responsiveness.
Hormonal Regulation and Ageing
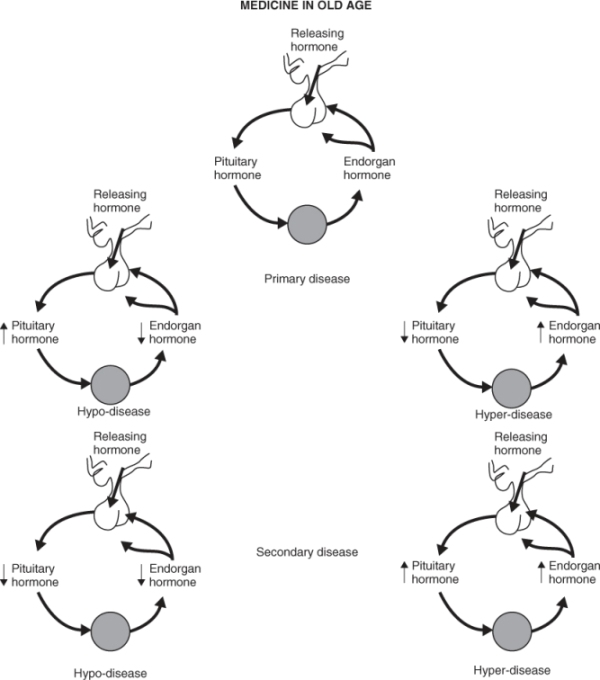
Hormones are regulated by a classical negative feedback system. Each peripheral hormone is regulated by a central system consisting of the hypothalamic–pituitary unit. The hypothalamus produces releasing hormones (and occasionally inhibitory hormones) that create a feedforward system that regulates the pulsatility and the circadian rhythm of hormone release. These releasing hormones regulate the release of anterior pituitary hormones, which in return result in the release of endorgan hormones. The endorgan hormones then feed back at the pituitary and the hypothalamic level to inhibit further release of pituitary hormones (Figure 95.1). When disease occurs in the endorgan hormone, it leads to failure of the endorgan and, therefore, negative feedback with an increase in the pituitary hormone (HYPO-disease) or increased activity of the endorgan with suppression of pituitary hormone release (HYPER-disease). When this occurs, the disease is considered to be primary endorgan disease, for example, primary hypothyroidism. Alternatively, failure can occur in the hypothalamic–pituitary unit, leading to a decrease in both the pituitary and the endorgan hormone, and this is known as secondary disease, for example, secondary hypogonadism. Finally, excess production of either a hypothalamic releasing hormone or pituitary hormone can occur. An example of this central form of HYPER-disease would be Cushing syndrome.
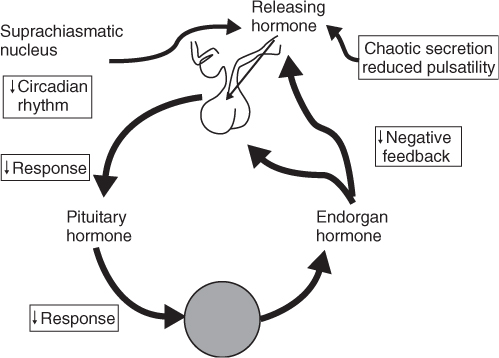
Ageing has effects on all levels of the hypothalamic–pituitary–gonadal axis. The circadian rhythm is controlled by the suprachiasmatic nucleus, which feeds information to the hypothalamus. The hypothalamic releasing hormones are responsible for maintaining the pulsatility of hormone release, which is essential for optimal hormonal action. With ageing, the pulse generator leads not only to a decline in maximal hormone production, but also to an irregular or ‘chaotic’ production of hypothalamic releasing hormones. This is amplified at the pituitary level where there is a decrease in the ability to respond to the hypothalamic signal. In addition, the endorgan itself has decreased responsiveness to the stimulus from the pituitary hormone1–4 (Figure 95.2).
In addition, changes in hormonal binding to its receptor and postreceptor responsiveness can also occur with ageing. An example is the posterior pituitary hormone, arginine vasopressin (AVP) or antidiuretic hormone (ADH). There is a decline in the renal responsiveness to AVP with ageing, which leads to an increase in basal secretion of AVP. A small further increase in AVP then puts the older person at high risk of developing hyponatraemia and syndrome of inappropriate ADH.5 There is also an attenuation of the normal increase in AVP that occurs at night. This increase is important for reabsorption of fluid during sleep and, therefore, its attenuation with ageing leads to increasing nocturia.6
Classically, endocrinologists have interpreted circulating hormone levels in the absence of an understanding of the functioning of the receptor. This is becoming less acceptable, as was shown recently by the example of the testosterone receptor and prostate cancer. The testosterone receptor contains a number of CAG repeats. The more the repeats, the less responsive the receptor is to testosterone. Prostate cancer occurs less often in males with a higher number of CAG repeats.7
Table 95.1 lists the hormonal changes seen with ageing. The levels of circulating hormones are determined by their production and clearance rates. Thus, the level of thyroxine remains normal because both the production and clearance rates decrease equally. Cortisol levels are slightly increased as there is a greater decrease in clearance rates. Cholecystokinin levels increase markedly due to the decline in clearance rate.8
Table 95.1 Hormonal changes associated with ageing.
| Decreased | Normal | Increased |
| Growth hormone | Estrogen (men) | ACTH |
| Insulin growth factor-1 | Luteinizing hormone (men) | Cholecystokinin |
| Testosterone | Thyroxine | Insulin |
| Estrogen (women) | Pancreatic polypeptide | Amylin |
| Dehydroepiandrosterone | Gastric inhibitory peptide | Luteinizing hormone (women) |
| Pregnenolone | TSH | FSH |
| Triiodothyronine | Glucagon-releasing peptide | Vasoactive intestinal peptide |
| 25-(OH)-vitamin D | Epinephrine | Cortisol |
| 1,25-(OH)2-vitamin D | Prolactin | Parathyroid hormone |
| Aldosterone | Norepinephrine | |
| Calcitonin | Glucagon |
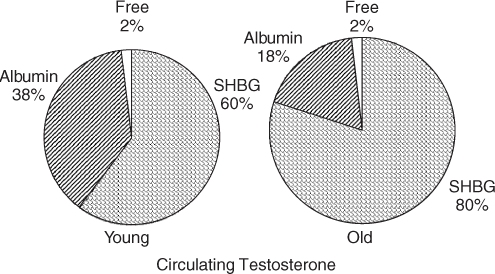
It is generally believed that free hormone or tissue-available hormone levels determine the effectiveness of the hormone. Thus, in the case of testosterone in males, there is a marked increase in sex hormone binding globulin (SHBG) with ageing. The testosterone bound to SHBG is thought not to be available to tissues (Figure 95.3). The rest of the testosterone is free or bound to albumin, which is thought to be tissue available. Hence measurement of the bioavailable testosterone gives a more accurate reflection of the true testosterone level than does a total testosterone measurement.9 Similarly, a number of growth hormone-binding proteins can produce marked changes in the ability of growth hormone to access its receptor.
Figure 95.3 Effect of altered binding proteins with ageing on the effect of tissue-available hormones: the example of testosterone.
Effects of Ageing and Related Diseases on Endocrine Diseases
With ageing, there is a blurring of the boundaries between health and disease. The age-related decline in many hormone levels results in difficulties in making the biochemical diagnoses of endocrine disorders. The decreased functional reserve of endocrine organs that occurs with ageing increases the propensity for older persons to develop endocrine deficiency disorders. With ageing there is a decrease in T-suppressor lymphocytes and an increase in autoantibodies. Many endocrine disorders are due to autoimmune disease and these changes amplify the possibility of an older person developing hypoendocrine disease in old age. There is also an increased likelihood of the development of polyglandular failure syndromes.
The decline in receptor and postreceptor responsiveness that occurs with ageing often leads to atypical presentations. Apathetic thyrotoxicosis is due, in part, to the decreased postreceptor responsiveness for β-adrenergic receptors that occurs with ageing. The classical changes of apathetic thyrotoxicosis include depression, weight loss, atrial fibrillation, heart failure, blepharatosis and proximal myopathy. Apathetic thyrotoxicosis occurs only in about 7% of older persons with hyperthyroidism. The presentations of endocrine disease in older persons are further confused by the fact that they often present with non-specific symptoms, for example, delirium, fatigue, falls, weight loss, cognitive decline or depression. These symptoms are common in older persons and can lead to delayed diagnosis. For example, Addison’s disease can present with weight loss, fatigue, abdominal pain, diarrhoea and hyponatraemia. The increase in cancer with ageing can lead to ectopic hormone production with an increase in endocrine disorders such as the syndrome of inappropriate ADH.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree