div class=”ChapterContextInformation”>
7. Reproductive Endocrinology
7.1 Reproductive Physiology: The Ovary and Testes—Anatomy and Physiology
The mature ovaries are paired nodular structures having 2.5–5/2/1 cm, while normal adult ♂ testicular volume is around 18.6 ± 4.8 mL.
The synthesis and secretion of sex steroids
Gametogenesis
The components of the ovarian follicle are the theca cells, the granulosa cells, and the primary oocyte. The theca cells produce androgens, and the granulosa cells produce estrogens. The other stromal cells that contribute to androgen production can be divided into the secondary interstitial cells (derived from theca) and the hilum cells.
The testes contain two major components that are structurally separated and serve different functions: the Leydig cells, or interstitial cells, comprise the major endocrine component and the seminiferous tubules that make up 90% of testicular volume.
Interstitial cells: ♂ Leydig cells, ♀ theca cells—which are found in between the seminiferous tubules and follicles, respectively. Produce androgens under LH drive.
Cells supporting gametogenesis: ♂ Sertoli cells, ♀ granulosa cells—secrete estrogens and other hormones, including inhibin and Müllerian inhibitory factor (AMH) under FSH drive. The former inhibits FSH secretion from the pituitary gland, and the latter–AMH, when secreted by Sertoli cells, is responsible for suppressing ♀ sex organ development during sexual differentiation in utero.
In adult ♀, AMH is produced in proportion to germ cell number and is, therefore, a marker of ovarian ageing.
The Sertoli cells also secrete androgen-binding protein (ABP), a molecule with high affinity for androgens. This substance, which enters the tubular lumen, provides a high concentration of testosterone to the developing germinal cells during the process of spermatogenesis.
Germ cells: ♂ continue to make new germ cells throughout adult life in the basal membrane of tubules. ♀ cease to make new germ cells after birth and are, therefore, born with all of the “eggs” that they will ever make.
♂ Seminiferous tubules. Spermatogenesis occurs here in the presence of high intratesticular concentrations of testosterone. Made up of germ cells and Sertoli cells that line the basement membrane and form tight junctions with other Sertoli cells, establishing a blood–testis barrier, through which spermatogonia mature to be released into the lumen of the tubule.
♀ Graafian follicle. Primordial follicles are recruited in batches, mature over 2 months, with selection of a dominant follicle with single central oocyte surrounded by granulosa cells which convert theca-derived testosterone to estradiol (under the influence of aromatase expressed by these cells) for ovulation. Follicles which do not proceed to ovulation become atretic.
7.1.1 The Regulation of Gonadal Function: The Hypothalamic-Pituitary-Gonadal Axis
7.1.1.1 Hypothalamus
Gonadotrophin-releasing hormone (GnRH) is secreted by the hypothalamus in a pulsatile manner (every 30–120 min) in response to stimuli from the cerebral cortex and limbic system via various neurotransmitters, for example, leptin, kisspeptin, endorphins, catecholamines, and dopamine.
GnRH release initially occurs during sleep in early puberty and then throughout the day in adulthood. It stimulates the secretion of LH and FSH by the pituitary gland.
The pattern of GnRH secretion is crucial for normal gonadotrophin secretion. Faster pulse frequencies are essential for LH secretion, whereas slower frequencies favor FSH secretion; continuous administration of GnRH abolishes both LH and FSH secretion.
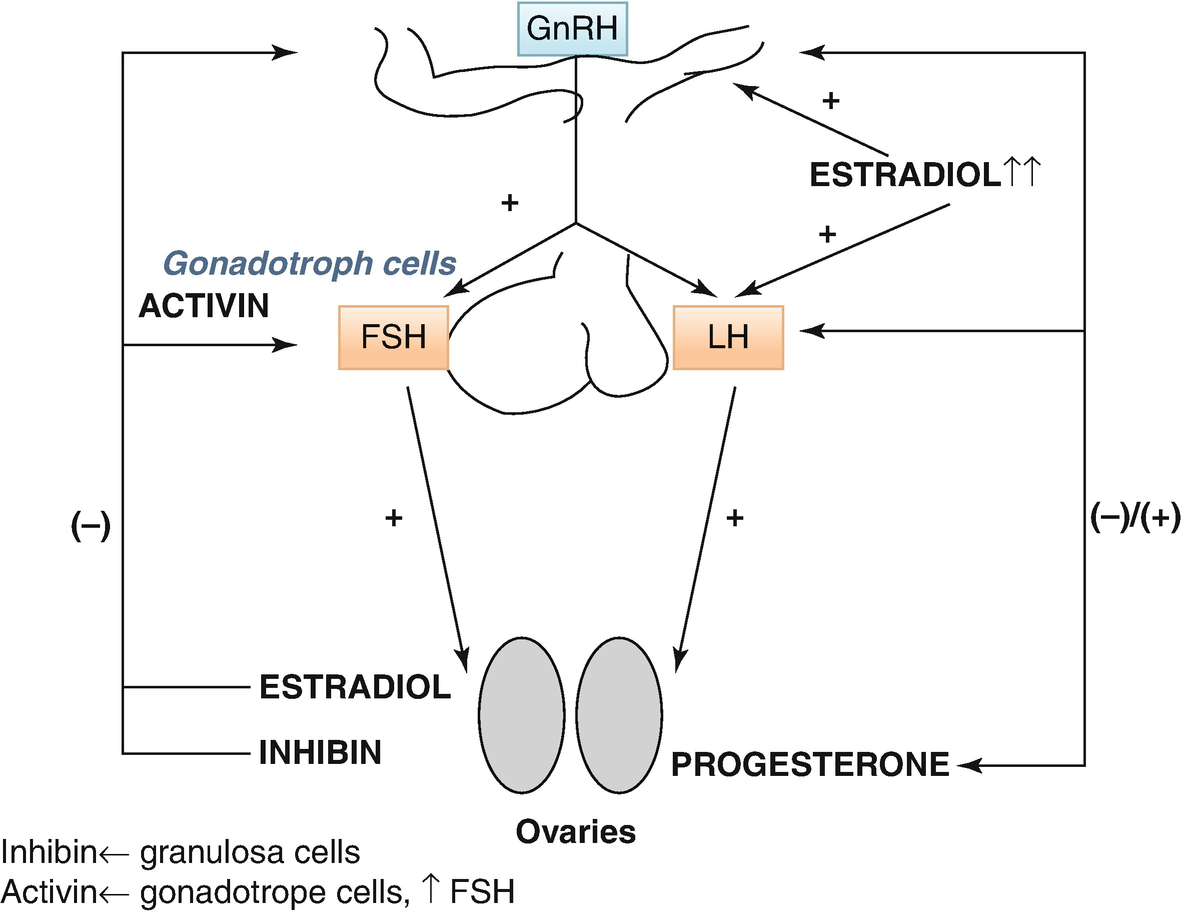
The hypothalamic–pituitary–ovarian (HPO) axis
7.1.1.2 Pituitary
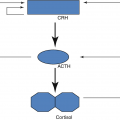
LH drives interstitial cell synthesis and secretion of testosterone (Fig. 7.2) in both sexes and triggers ovulation in ♀. Negative feedback by sex steroids.
FSH in ♂ drives Sertoli cell-mediated sperm maturation and production of seminiferous tubule fluid as well as a number of substances thought to be important for spermatogenesis and inhibin. FSH in ♀ drives granulosa cell-mediated aromatization of androgens to estradiol. Negative feedback mainly by inhibin (+ steroids). An intrapituitary network involving several factors, including activin that enhances FSH, plays a role in regulating gonadotropin synthesis and secretion (Fig. 7.1).
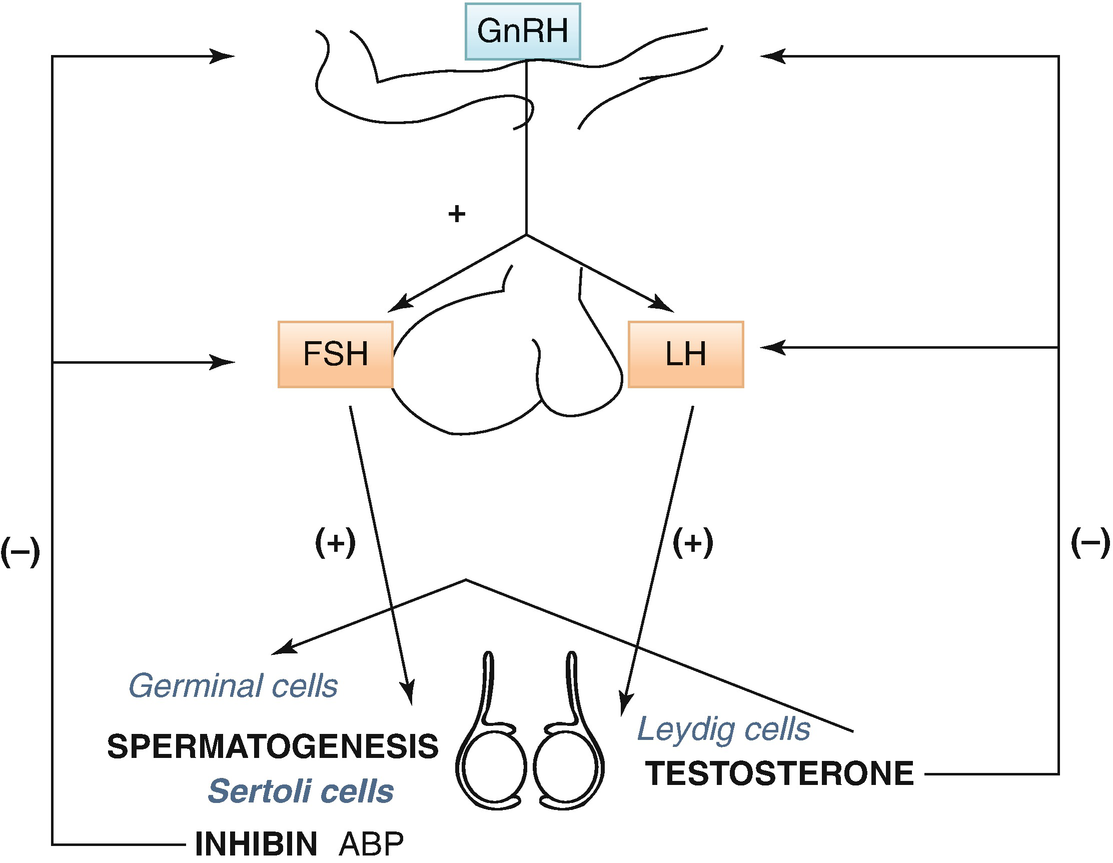
The hypothalamic–pituitary–testicular axis
7.1.1.3 The Gonads Hormones
The Ovary
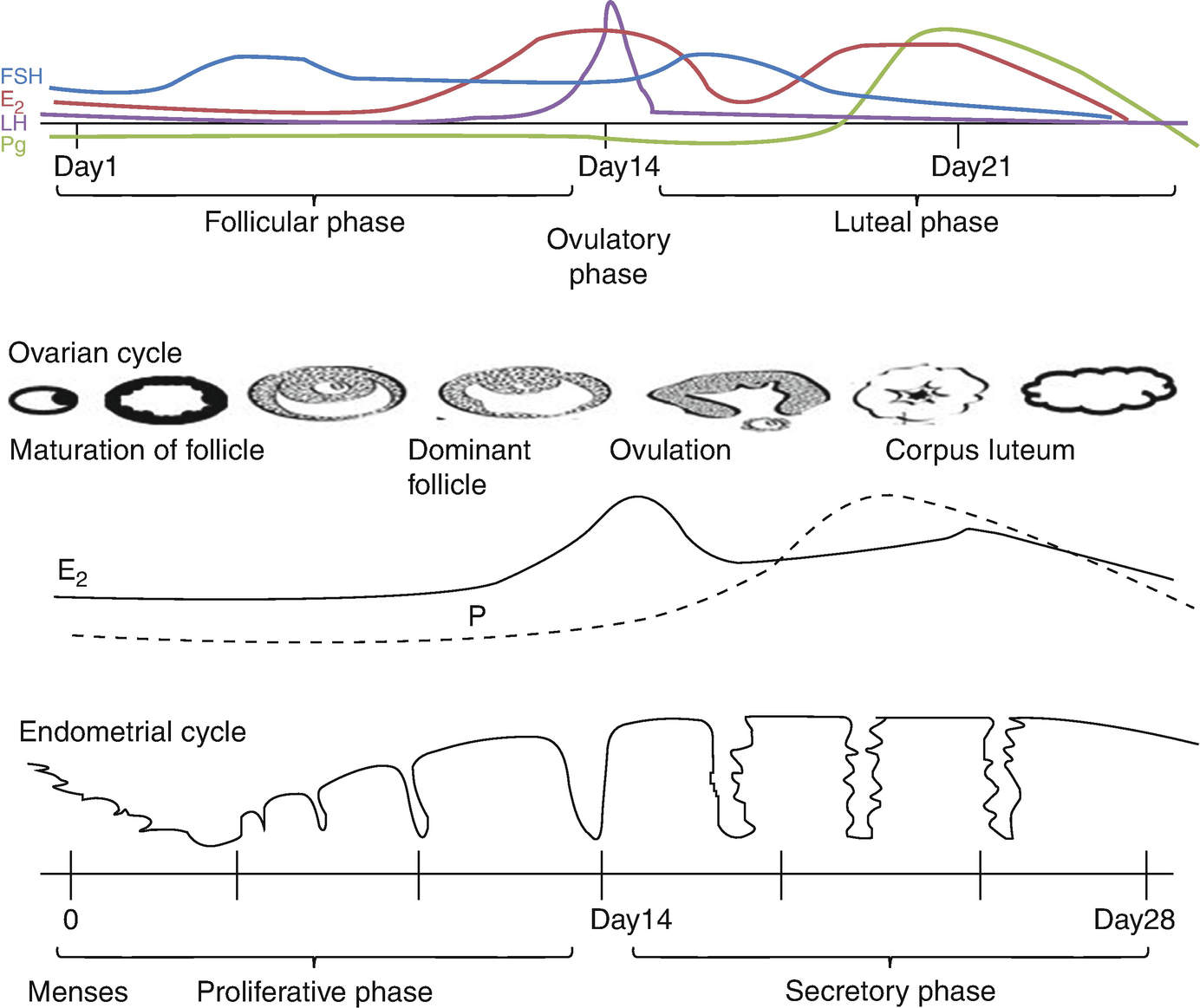
Menstrual cycle
Progesterone secreted from corpus luteum (during the luteal phase), which is derived from granulosa cells, only after ovulation occurs. Peak production on day 21 of a 28-day cycle.
Ovaries also secrete testosterone and other androgens which either serve as precursors to estrogen synthesis—the theca cells, which lack aromatase, synthesize androgens (mainly androstenedione) which diffuse into the granulosa cells (which lack 17 hydroxylase) where they are used for estrogen production or are released into the circulation to act on peripheral tissues.
The Testes
In ♂, testosterone and small amounts of DHEA sulfate and DHT are produced by Leydig cells. ♂ testosterone has a circadian rhythm, with maximum secretion at around 8 a.m. and minimum around 9 p.m.
7.1.2 Regulation of Gametogenesis
Both FSH and LH are required for gametogenesis.
FSH, through its action on Sertoli and granulosa cells, is vital for sperm and oocyte maturation.
FSH is necessary for the initiation of spermatogenesis; however, full maturation of the spermatozoa appears to require not only an FSH effect but also testosterone.
LH ensures high intragonadal concentrations of testosterone (exogenous testosterone cannot substitute).
♂: the whole process of spermatogenesis (spermatogonia → I spermatocytes → II spermatocytes → spermatids → spermatozoa) takes approximately 74 days, followed by another 12–21 days for sperm transport through the epididymis.
♀: from primordial follicle to primary follicle, it takes about 180 days (a continuous process). It is then another 60 days to form a preantral follicle which then proceeds to ovulation three menstrual cycles later. Only the last 2–3 weeks of this process is under gonadotrophin drive, during which time the follicle grows from 2 to 20 mm.
7.1.3 Androgen Action
Both testosterone and DHT exert their activity by binding to androgen receptors, the latter more avidly than testosterone.
♂ sexual differentiation during embryogenesis.
Development and maintenance of ♂ sex characteristics after puberty.
Normal ♂ sexual function and behavior.
Spermatogenesis.
Regulation of gonadotrophin secretion.
7.1.4 Estrogen Action
Estradiol binds to A (reproductive tissues) and B (bone, brain, heart, etc.) receptors.
Development of ♀ sex characteristics.
⊕ stromal development and ductal growth in the breast.
Increase fat stores.
Increase vaginal wall and uterine thickening.
Cervix: ⊕ production of large amounts of thin watery mucus, through which sperm can penetrate most readily.
7.1.5 Progesterone Action
Converts the endometrium to its secretory stage to prepare the uterus for implantation.
Affects the vaginal epithelium and cervical mucus, making it thick and impenetrable to sperm.
Glandular development of the breasts.
↑ the body temperature in humans.
7.1.6 Menstrual Cycle
The ovary has two phases: the follicular phase and the luteal phase. The endometrium has three phases and is synchronized by the ovary. The complex feedback loops between the ovary and the HP axis regulate the menstrual cycle. During the follicular phase, the ovary secretes estradiol, which stimulates the endometrium to undergo proliferative phase. After ovulation (luteal phase) the ovary secretes estrogen and progesterone, which maintains the endometrial lining and promotes the secretory phase. In a nonpregnant cycle, luteolysis occurs, resulting in cessation of hormone production. This hormone withdrawal results in the degenerative phase and the onset of menses (Fig. 7.3).
7.2 Menstrual Function Disorders: Assessment and Investigation
7.2.1 Definitions
Primary amenorrhea = failure of menarche by the age of 16 years.
Secondary amenorrhea = the cessation of menses for >6 months in ♀ who had previously menstruated or no menses for more than three cycles in an individual who has previously had cyclic menses.
Oligomenorrhea is defined as the reduction in the frequency of menses to <9 periods a year.
The causes of amenorrhea are grouped according to the level of involvement in the regulatory system that governs normal menstrual activity (i.e., hypothalamic, pituitary, ovarian, and uterine). If the outflow tract is intact (uterus and vagina present), amenorrhea is most likely the result of disruption in the HPO axis.
7.3 Ovarian Insufficiency or ♀ Hypogonadism
7.3.1 Definition
Ovarian insufficiency also termed hypogonadism is defined as diminished functional activity of the ovaries—that may result in diminished production of sex hormones.
7.3.2 Classification of ♀ Hypogonadism (Ovarian Insufficiency)
Secondary/central hypogonadism (this is usually caused by diseases of the pituitary gland or hypothalamus), also referred to as hypogonadotropic hypogonadism.
Primary/peripheral hypogonadism (means that the ovaries do not function normally), also known as ovarian failure (it is diagnosed based on amenorrhea, ↑ FSH > 40 UI/L. This may occur at any time from embryonic development onward. If it occurs prior to age 40, it is called premature ovarian failure or insufficiency (POF/POI)).
Note: POI/POF is a disorder characterized by amenorrhea, estrogen deficiency, and elevated gonadotrophins, developing in ♀ <40 years, as a result of loss of ovarian follicular function.
before puberty
after puberty
Polycystic ovary syndrome
Hypothalamic amenorrhea
Hyperprolactinemia
Ovarian insufficiency
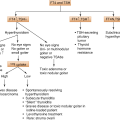
Classification of amenorrhea and ovarian insufficiency
Amenorrhea + Absent breast development (or ♀ hypogonadism with prepubertal onset) |
Primary ovarian insufficiency • Gonadal agenesis • Gonadal dysgenesis (45 XO: Turner syndrome and its variants: mosaicism (e.g., X/XX, X/XX/XXX)) • 46,XX or 46,XY (Swyer syndrome) pure gonadal dysgenesis • Defects in estrogen biosynthesis (e.g., CYP17 α-deficiency, see Fig. 6.1) |
Secondary ovarian insufficiency (see also chapter pituitary insufficiency) • Inadequate GnRH synthesis (Kallmann syndrome) • Developmental anatomic abnormalities in the central nervous system • Constitutional delay in puberty • CNS neoplasm (craniopharyngioma, gliomas) • Isolated gonadotropin insufficiency • Pituitary tumors • Pituitary insufficiency • Prepubertal hypothyroidism |
Amenorrhea + Breast development (or ♀ hypogonadism with postpubertal onset) |
Primary ovarian insufficiency POF • Iatrogenic (radiation, chemotherapy) • Mosaicism (46,XX/XO,XX/XY) • Infections (e.g., mumps) • Autoimmune destruction • Fragile X syndrome (Fragile X premutations) (an association has been described between a distal mutation on the long arm of the X chromosome—in the FMR1 gene (fragile X gene)—the FMR1 permutation state and premature ovarian failure) Resistant ovary syndrome (a rare cause of hypergonadotropic amenorrhea associated with numerous unstimulated ovarian follicles; the literature to date is inconclusive about the cause) |
Secondary ovarian insufficiency Hypothalamic etiology • Functional hypothalamic amenorrhea Stress Amenorrhea associated with eating disorders (anorexia nervosa, bulimia) Amenorrhea associated with strenuous exercise • CNS neoplasm • Chronic disease Pituitary etiology • Hyperprolactinemia and pituitary tumors • Acquired pituitary insufficiency (see also chapter pituitary failure) |
7.3.2.1 Functional Hypothalamic Amenorrhea
Functional hypothalamic amenorrhea is one of the most common types of amenorrhea and accounts for 15–35% of cases. It is an endocrine disorder, although the exact mechanism has not been definitively determined. It is characterized by a reduced GnRH drive (decrease in pulse frequency and amplitude), leading to low or low-normal serum levels of FSH and LH and resulting in anovulation.
The ratio of serum FSH to LH in these patients is often equivalent to that of a prepubertal female with a relative FSH dominance (FSH-LH ratio >1 in the presence of hypoestrogenemia).
Hypothalamic dysfunction has been observed in female athletes. Competitors in events such as gymnastics, ballet, marathon running, and diving can show menstrual irregularities ranging from luteal phase defects to amenorrhea.
Hypothalamic dysfunction and functional hypothalamic amenorrhea have been described also in association with eating disorders. Anorexia nervosa is a disorder characterized by relentless dieting in pursuit of a thin body habitus. Approximately 95% of cases occur in females, and the onset is chiefly in adolescence. The clinical features include extreme weight loss leading to a body weight less than 85% of normal for age and height, a distorted body image, intense fear of gaining weight, a preoccupation with food, with an obsessive-compulsive personality. The associated symptoms include hypothermia, mild bradycardia, dry skin, constipation, and symptoms of hypoestrogenemia. Bulimia occurs in about half of patients with anorexia and is defined as binge eating followed by self-induced purging. These patients also have a variety of neuroendocrine aberrations—often to a lesser degree than those with anorexia—which also lead to menstrual disturbances.
History and Physical Examination
Manifestations of estrogen deficiency, for example, hot flushes, reduced libido, and dyspareunia.
Hypothalamic dysregulation, for example, exercise and nutritional history, body weight changes, BMI, emotional stress, recent or chronic physical illness.
In primary amenorrhea—history of breast development, history of cyclical pain, age of menarche of mother and sisters.
In secondary amenorrhea—duration and regularity of previous menses, family history of early menopause or familial autoimmune disorders.
Secondary sex characteristics.
Anosmia may indicate Kallmann’s syndrome.
Hirsutism or acne, evidence of hyperandrogenism or virilization.
Galactorrhea, visual field defects.
History or evidence suggestive of pituitary, thyroid, or adrenal dysfunction.
Drug history—for example, causes of hyperprolactinemia, chemotherapy, hormonal contraception.
Obstetric and surgical history.
Features of Turner’s syndrome or other dysmorphic features.
7.3.3 Clinical Features in ♀ Hypogonadism
Ovarian insufficiency WITH PREPUBERTAL ONSET → poor sexual secondary sexual development and eunuchoid skeletal proportions |
SEXUAL INFANTILISM Primary amenorrhea Absence/sparse of pubic and axillary hair Absence of breast development (thelarche) Infantile aspect of external and internal genitalia SOMATIC MODIFICATIONS Tall stature, eunuchoid skeletal proportions (crown to pubis to pubis to floor ratio <1) Low peak bone mass PSYCHOLOGICAL DISTURBANCES ↓ libido |
Ovarian insufficiency WITH POSTPUBERTAL ONSET: |
• Secondary amenorrhea • Infertility • Decrease in axillary and pubic hair • Atrophy of breast • Involution of genital tract, urogenital atrophy → vaginal dryness and pain with intercourse, atrophic cystitis • Precocious appearance of wrinkles • Vasomotor symptoms (hot flushes) • Late complications: osteoporosis, atherosclerosis with ↑ risk of cardiovascular and cerebrovascular disease |
7.3.4 Workup of ♀ Hypogonadism
- 1.
Firstly, rule out thyroid dysfunction and hyperprolactinemia (check: TSH, FT4, PRL).
- 2.
Is it primary or secondary ovarian dysfunction/insufficiency?
Measure FSH, LH, E2
↓/low-normal FSH, LH + ↓ E2 = secondary (central) ovarian insufficiency/hypogonadotropic hypogonadism with prepubertal onset
In these patients, LH and FSH responses to testing with GnRH may help to differentiate delayed puberty from a more serious problem (e.g., craniopharyngioma).
Do hormonal, neuroradiologic and neuro-ophthalmologic assessment of hypothalamus and pituitary-MRI of the pituitary fossa (e.g., to rule out tumors: craniopharyngiomas).
↑ FSH, LH + ↓ E2 = primary ovarian insufficiency/hypergonadotropic hypogonadism
If the patient is short in stature and has obvious stigmas of Turner’s syndrome → Barr test, Karyotype (45X0).
If the patient is of normal height or has relatively longer arms and legs compared with the length of the trunk (eunuchoid proportions) → search for other causes of primary ovarian insufficiency: autoimmune destruction (do anti-ovary autoantibodies), exclude anatomical abnormalities (ovarian and uterine morphology: do sonography, celioscopy with biopsy for gonadal agenesis) (Fig. 7.4).
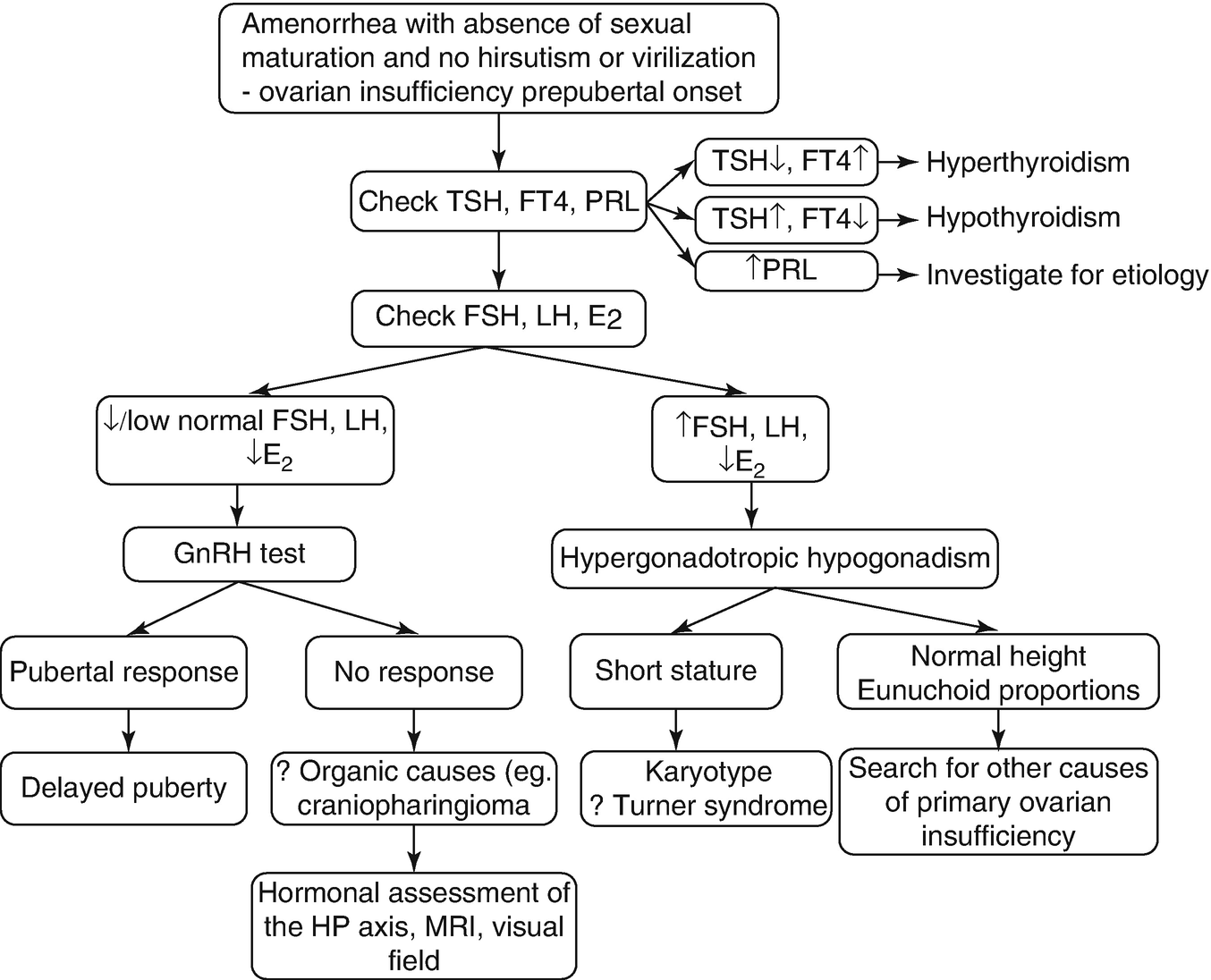
Workup of primary amenorrhea with absence of sexual maturation
! The possibility of pregnancy should always be considered (do a pregnancy test!).
→ Localize the underlying cause of amenorrhea: dysfunction of either the outflow tract or disruption in the HPO axis
Thyroid dysfunction? Hyperprolactinemia? → Measure: TSH, FT4, PRL concentrations; if ↑ PRL: search for a cause of hyperPRL: prolactinoma/“disinhibition” hyperPRL, etc.
If TSH, FT4, PRL are normal →
Assess endogenous estrogen production—indirectly (do progesterone withdrawal test).
Induce withdrawal bleed with progesterone (e.g., 10 mg medroxyprogesterone acetate bd for 7–10 days or dydrogesterone 10–20 mg/day daily for 5–10 days).
→ If a bleed occurs within about 1 week after the last pill (“withdrawal bleeding”), then there is adequate estrogen priming and endometrial development (positive test).
→ If no signs of hyperandrogenism are present, then, the ♀ patient does not have a serious problem ⇒ just reassurance.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree