Hypothalamic-pituitary-gonadal axis
Reproductive endocrinology includes the hormones of the hypothalamic-pituitary-gonadal axis and the adrenal glands. The hormones necessary for proper reproductive function include gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH), follicle-stimulating hormone (FSH), and various sex steroids. Steroids that feminize are classified as estrogens, and those that masculinize are known as androgens. These steroids are produced by the ovaries, testes, and adrenal glands.
Female reproductive disorders
The complexity of the female reproductive system allows the opportunity for many abnormalities to occur ( Table 5.1 ). Included here are prepubertal disorders, hirsutism, irregular menses, and polycystic ovarian syndrome (PCOS). All of these disorders can affect fertility, and that will be discussed here in a separate infertility section.
| Hypogonadotropic hypogonadism |
| Pituitary |
| Necrosis |
| Adenoma |
| Inflammatory disorders |
| Hypothalamus |
| GnRH defect (chronic stress, malnutrition, Kallmann syndrome, strenuous exercise) |
| Tumors |
| Infection |
| Hypergonadotropic hypogonadism |
| Premature ovarian failure |
| Chronic anovulation (PCOS, adrenal disease, thyroid disease, ovarian tumor) |
| Gonadal agenesis |
| Gonadal dysgenesis |
| Steroidogenesis deficiency (17-hydroxylase deficiency, 17,20-lyase deficiency) |
Prepubertal disorders
Puberty occurs in a predictable sequence of events, with breast development appearing first, followed by growth of pubic hair, a linear growth spurt, and finally menarche . Puberty is initiated by the release of GnRH by the hypothalamus, which stimulates secretion of LH and FSH by the pituitary. The normal age for onset of puberty for females is between 8 and 13 years . Precocious puberty is the development of secondary sexual characteristics before the age of eight and is 10 times more common in girls than boys. Nonpathologic variants of precocious puberty include premature thelarche (premature breast development) and premature adrenarche (premature sexual hair development). When these signs are present individually and not in conjunction with increased rates of bone growth, they are usually not indicative of precocious puberty, and further investigation may not be warranted .
Pathologic precocious puberty is either GnRH-dependent, known as central precocious puberty (CPP), or GnRH-independent, which is referred to as peripheral precocious puberty (PPP). The majority of cases of CPP in girls are idiopathic. Known causes of CPP include central nervous system tumors such as hypothalamic hamartomas, hydrocephalus, neurofibromas, neoplasms, and trauma. The gold standard for the diagnosis of CPP is still the GnRH stimulation test, even with the increases in sensitivity and accuracy with LH testing . A peak serum LH concentration between 5 and 8 IU/L after GnRH administration is considered diagnostic for precocious puberty . It is also useful to compare the ratio of FSH concentration with LH concentration after stimulation, and a ratio of greater than 0.66 is indicative of a pubertal response . Treatment of CPP includes the use of GnRH agonists to inhibit gonadotropin release and delay pubertal progression .
In girls with PPP, the secretion of sex steroids is independent of pituitary gonadotropin release; the response to the GnRH stimulation test is repressed, and concentrations of LH and FSH are low. The increase in sex steroids could be caused by a gonadal or adrenal tumor, hepatoblastoma, hypothyroidism, McCune–Albright syndrome (MAS), or congenital adrenal hyperplasia (CAH). Of the cases of CAH, 95% are due to 21-hydroxylase (21-OH) deficiency, which results in cortisol deficiency and androgen excess . Classic cases of CAH present with virilization, growth acceleration, and accelerated bone maturation. Basal, early morning 17-hydroxyprogesterone concentrations measured via liquid chromatography-mass spectrometry can be used to screen for 21-OH-deficient CAH. 17-Hydroxyprogesterone is the substrate of 21-OH, and high concentrations will be present when 21-OH is nonfunctional. The gold standard for diagnosis of this disorder is the adrenocorticotropic hormone (ACTH) stimulation test, with a diagnostic cutoff for 17-hydroxyprogesterone of >1000 ng/dL (30 nmol/L) poststimulation .
Delayed puberty is defined as no signs of breast development by age 13 . Constitutional delay of growth and puberty is the most common cause of delayed puberty, and the majority of patients have a family history of a parent with delayed puberty. Constitutional delay of puberty is a diagnosis of exclusion. Other causes of delayed puberty can be diagnosed by measuring serum LH, FSH, and estradiol. Patients with hypergonadotropic hypogonadism have increased concentrations of LH and FSH compared to the pubertal range. Hypergonadotropic hypogonadism may be congenital or acquired, and is more commonly found in girls as opposed to boys. Hypogonadotropic hypogonadism results in low levels of LH and FSH as compared to the pubertal range and can be classified as functional or persistent. Patients with the persistent form will need hormonal treatment to induce puberty .
Hirsutism
Hirsutism is defined as the excessive growth of terminal hair in women and children in a male-like pattern and is found in 70%–80% of patients with hyperandrogenism . True hirsutism (thick and coarse terminal hair growth in androgen-responsive areas) must be distinguished from hypertrichosis (excessive growth of nonandrogen-responsive hairs). Women with androgen-responsive hirsutism either have excess androgen or have an increased sensitivity to normal concentrations of androgens. Not all hirsute patients have evidence of androgen excess or endocrine imbalance; this is termed idiopathic hirsutism and can be due to their ethnic background or an increase in 5α-reductase activity in the skin . PCOS is the most common cause of androgen hypersecretion, and 70%–80% of hirsute women also have PCOS . Nonclassical congenital adrenal hyperplasia (NCCAH), acromegaly, hyperprolactinemia, menopause, and ACTH-dependent Cushing syndrome can also be causes of hirsutism.
The 2018 clinical practice guideline on evaluation and treatment of hirsutism by the Endocrine Society suggests testing for elevated androgen concentrations in all women with an abnormal Ferriman–Gallwey hirsutism score or in women with patient-important hirsutism and menstrual irregularities . Serum total testosterone should be measured, followed by serum-free testosterone, in women with moderate to severe hirsutism who have normal total testosterone. Measurement of testosterone in women with automated laboratory immunoassays is problematic as these assays lack sufficient sensitivity to accurately and precisely quantify the low concentrations found in women. Measurement of serum testosterone in women is currently best assessed by liquid chromatography-tandem mass spectrometry or other technology that is sufficiently precise at these low concentrations. Women who are hyperandrogenemic should also be screened for NCCAH by measuring 17-hydroxyprogesterone. Women with a high risk of NCCAH should also be screened, even if they have normal total and free testosterone concentrations . The laboratory evaluation for hirsutism may also include measurement of dehydroepiandrosterone sulfate (DHEA-S) . If DHEA-S concentrations are elevated, it suggests an adrenal source of androgens, whereas an increase in testosterone suggests either an ovarian or an adrenal source. If circulating concentrations of testosterone are greater than 200 ng/dL, and DHEA-S concentrations are greater than 700 µg/dL, the possibility of an androgen-producing tumor should be evaluated. In the majority of cases, the most likely causes of hyperandrogenemia in women are PCOS or hyperthecosis . Oral contraceptives are recommended as first-line therapy for hirsutism, with an antiandrogen added after 6 months of treatment if patient-important hirsutism remains . Insulin-lowering drugs are not recommended for the treatment of hirsutism when hirsutism is the only clinical indication.
Idiopathic hirsutism is a diagnosis of exclusion in women with mild hirsutism, but normal ovulatory function and normal androgen concentrations .
Irregular menses
Menstruation occurs, on average, every 28 days in a normal ovulatory menstrual cycle. Amenorrhea, the absence of menstrual bleeding, can be characterized as either primary (never having a menstrual period) or secondary (menstruation is present and then ceases) ( Table 5.2 ). Pregnancy should always be ruled out first when dealing with both primary and secondary amenorrhea. Along with pregnancy, all conditions that can cause secondary amenorrhea can also cause primary amenorrhea, but the reverse is not the case .
| Primary amenorrhea |
| Pituitary-hypothalamic disorders |
| . Turner syndrome |
| Hypopituitarism |
| Kallmann syndrome |
| Nutritional disorders |
| Thyroid disorders |
| Hypothyroidism |
| Adrenal disorders |
| Congenital adrenal hyperplasia |
| Lower tract defects |
| Vaginal aplasia |
| Ovarian disorders |
| Testicular feminization |
| Polycystic ovarian syndrome |
| Uterine disorders |
| Endometritis |
| Müllerian agenesis |
| Secondary amenorrhea |
| Pregnancy/lactation |
| Adrenal disorders |
| Cushing syndrome |
| Ovarian disorders |
| Ovarian tumors |
| Polycystic ovarian syndrome |
| Premature ovarian failure |
| Uterine disorders |
| Asherman syndrome |
| Hypothalamic disorders |
| Tumors |
| Excessive exercise |
| Nutritional disorders |
| Stress |
| Pituitary disorders |
| Sheehan syndrome |
| Thyroid disorders |
| Hypothyroidism |
| Hyperthyroidism |
| Adrenal disorders |
| Nonclassical congenital adrenal hyperplasia |
| Cushing syndrome |
| Tumors |
| Iatrogenic |
| Antidepressants |
| Drugs with estrogenic activity |
Primary amenorrhea is defined as a failure to establish spontaneous periodic menstruation by the age of 15 or within 5 years of breast development if breast development occurs before the age of 10 . There is some controversy about this age limit because some studies suggest that it should be lowered . A physical examination, patient history, and laboratory evaluation of thyroid-stimulating hormone (TSH), FSH, and prolactin can identify the most common causes of amenorrhea . If there are high concentrations of FSH and LH in conjunction with low Tanner stages, this can be a sign of delayed puberty and hypergonadotropic hypogonadism. There are numerous causes of primary amenorrhea including Turner syndrome, androgen insensitivity syndrome, Müllerian agenesis, and hypogonadotropic hypogonadism, among others .
The most common cause for hypergonadotropic hypogonadism in females is Turner syndrome, which is defined as a 45,X karyotype and female phenotype . Moderately high concentrations of FSH and LH are also found in cases of androgen insensitivity syndrome, in which the patient has a 46, XY karyotype but is phenotypically female. Androgen insensitivity is characterized by the development of a vagina, absence of the ovaries and uterus, and presence of testes in the abdominal cavity. Approximately 10% of cases of primary amenorrhea are due to Müllerian agenesis. In Müllerian agenesis the Müllerian ducts fail to develop and there is a consequent absence of the uterus and variable hypoplasia of the upper vagina. In addition to physical differences, Müllerian agenesis can be distinguished from androgen insensitivity by evaluation of testosterone concentrations. In androgen insensitivity, testosterone will be in the male reference interval or higher, whereas in Müllerian agenesis, testosterone will be in the female reference interval . Patients with hypogonadotropic hypogonadism present with low concentrations of FSH, LH, and estradiol Kallmann syndrome (KS) is one example of hypogonadotropic hypogonadism and is discussed further in the section on male reproductive disorders.
Secondary amenorrhea is defined as the absence of three or more consecutive menstrual periods at some point after the first menstrual period. Oligomenorrhea is infrequent menstruation, occurring less than nine times a year . When investigating secondary amenorrhea, the laboratory testing should be guided by the presence or absence of signs of hyperandrogenism such as hirsutism, acne, and rapid weight gain. Concentrations of testosterone and DHEA-S higher than 200 and 700 µg/dL, respectively, are suggestive of adrenal or ovarian tumors . Slightly lower concentrations are suggestive of PCOS, which is discussed in the next section. In patients without signs of hyperandrogenism, elevated concentrations of FSH and LH could indicate ovarian failure. Premature ovarian failure is the failure of estrogen production in the setting of high gonadotropin concentrations when the patient is younger than 40 years of age . Ovarian failure can be confirmed by an FSH concentration persistently in the menopausal range. An MRI to assess the presence of a pituitary tumor should be performed after hypothyroidism is ruled out . Elevated concentrations of prolactin can also cause amenorrhea, and menses can resume once the prolactin concentrations are reduced by the preferred treatment of dopamine agonists, both in the presence or absence of a pituitary tumor . Other disorders of the uterus, pituitary, and hypothalamus can also cause amenorrhea. If the cause of amenorrhea has not been elucidated, serum estradiol should be measured, or a progesterone challenge can be done to determine relative estrogen status. Treatment with progesterone will stimulate menstrual bleeding in women with an estrogen-primed uterus. This so-called “withdrawal bleeding” (called this because bleeding begins when progesterone treatment is ceased) will occur as long as the estrogen concentration is adequate, and there is no outflow obstruction. The progesterone challenge test is preferred to measuring serum estradiol due to the daily fluctuations in estradiol concentrations, but can have a false-positive rate of about 20% .
Polycystic ovarian syndrome
PCOS is the most common endocrine disorder of reproductive-aged women, affecting approximately 10% of this population . PCOS is an endocrine disorder that presents with a spectrum of symptoms that may include hyperandrogenism, ovulatory dysfunction, the presence of polycystic ovarian morphology on ultrasound, menstrual cycle disturbances, anovulatory infertility, insulin resistance, and obesity . PCOS is also associated with an increased risk of endometrial cancer . The term “polycystic ovary” is actually a misnomer as the ovaries are not cyst filled, but rather are studded with numerous ovarian follicles arrested in the small antral follicle stage of development.
The diagnosis of PCOS is currently made based on the Rotterdam diagnostic criteria that require that for adult women two of the following three criteria be met: (1) The patient must demonstrate oligo- or anovulation; (2) there must be clinical and/or biochemical signs of hyperandrogenism; (3) demonstration of polycystic ovaries via ultrasound. Importantly, other causes of these features such as hyperprolactinemia, thyroid disease, and others must also be absent . For adolescents (<20 years of age) the diagnostic criteria are slightly different in that the use of ultrasound is not recommended and a minimum of 2 years must have passed since menarche .
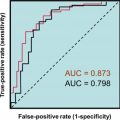
The assessment of androgen excess does not rely on laboratory testing and be done via the observation of hirsutism and female pattern hair loss. Laboratory assessment of the androgen excess may include evaluation of serum testosterone, androstenedione, LH, and FSH or the calculation of the free androgen index . Testosterone and androstenedione are frequently elevated in PCOS; however, there is a marked overlap in the distribution of values between women with and without PCOS. In PCOS, the concentrations of LH are often elevated, while FSH can be low-to-low-normal, for this reason some have proposed the use of an elevated LH/FSH ratio to indicate PCOS. For all these proposed serum markers there is considerable overlap in the distributions between women with and without PCOS. Consequently, the area under the curve (AUC) from receiver operator characteristic analyses are quite low with the AUC of testosterone equal to 0.75, androstenedione 0.66, and LH/FSH ratio equal to 0.72 .
It is also important to recall that while PCOS is a disorder characterized by hyperandrogenism that patients with PCOS will also typically present with high estradiol due to the peripheral aromatization of testosterone.
Recently there have been a number of publications that have explored the use of anti-Müllerian hormone (AMH) as a diagnostic marker of PCOS. In women, AMH is produced primarily by the small antral follicles of the ovary, which is the stage at which follicles are arrested in PCOS. Consequently, serum AMH concentrations in women with PCOS are higher than in non-PCOS women and AMH concentration correlates better with the antral follicle count than do other serum biomarkers such as FSH, LH, estradiol, or inhibin B . AMH as of yet has not been adopted into the diagnostic criteria for PCOS, partially because of the need for assay harmonization . Other challenges to the use of AMH that will need to be addressed is the need for age-specific thresholds as AMH normally decreases with age beginning at approximately the age of 25. AMH concentrations are affected by body mass and smoking with lower concentrations observed in overweight and obese women, as well as in women who smoke .
Infertility
Infertility is defined as the inability to conceive after 1 year of unprotected intercourse. Age is one of the main factors in a woman’s ability to conceive, and beyond the age of 35, the chances of conceiving naturally as well as the successful outcome of treatment strategies decrease dramatically . If a couple has not conceived after 1 year of unprotected sexual intercourse (6 months if the woman is over 35), testing should be undertaken to try to determine the nature of the problem. One approach to the evaluation of female infertility is shown in Fig. 5.1 . Factors that can contribute to female infertility include ovulatory dysfunction, ovarian reserve, and structural factors.
| Female infertility evaluation |
History
|
Physical exam
|
Ovulatory function
|
| Uterine abnormalities |
| Tubal patency |
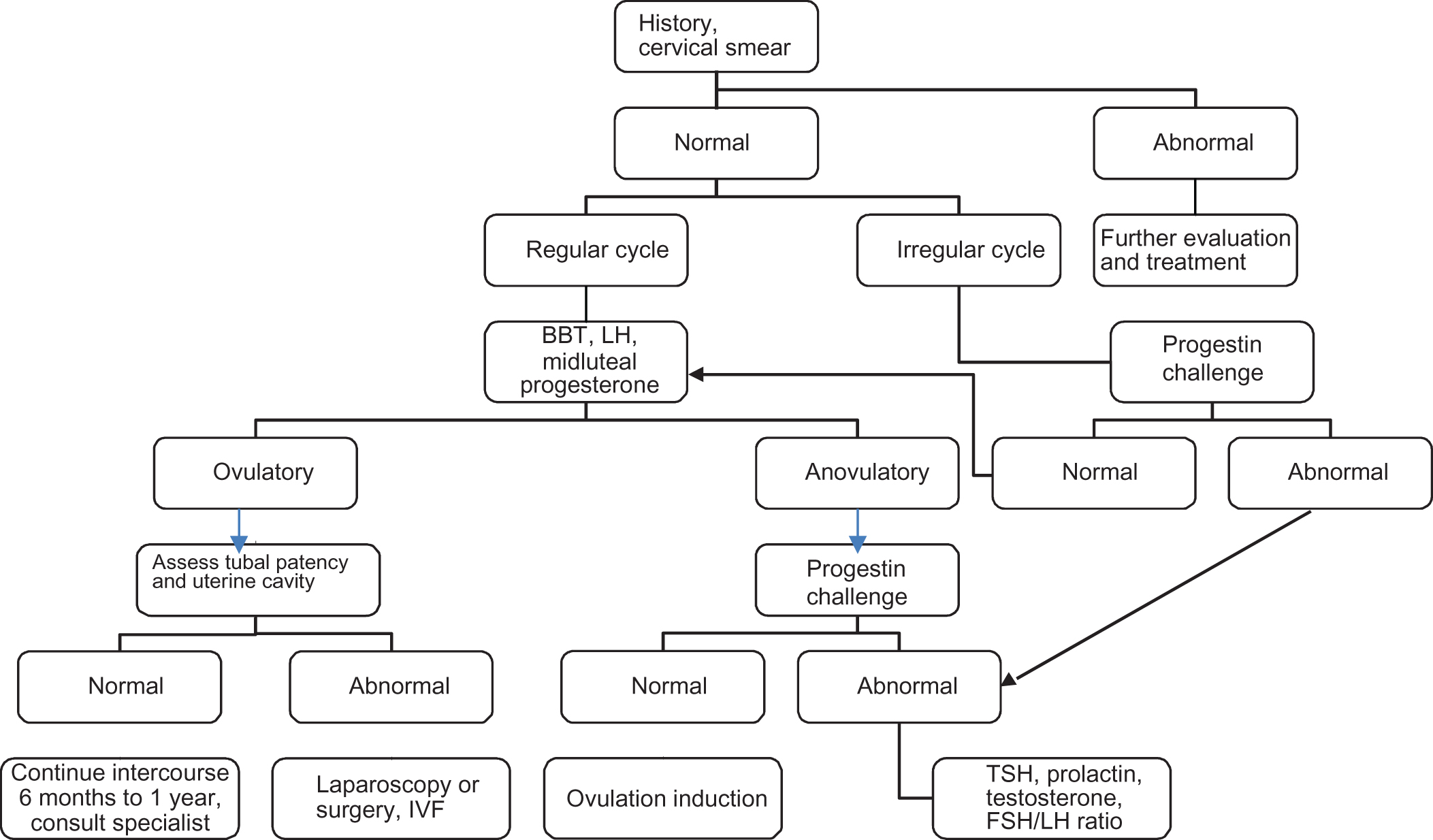
Ovulatory dysfunction
Disorders of ovulation account for up to 30% of infertility and can manifest as either oligomenorrhea or amenorrhea . PCOS is the most common cause of anovulation and has been discussed earlier in this chapter. Obesity, hypothalamic and pituitary dysfunction, hyperprolactinemia, and thyroid disorders can also cause ovulatory dysfunction.
Current laboratory tests do not directly confirm ovulation, but the measurement of serum progesterone serves as a good indicator. The increase in progesterone that occurs during the midluteal phase (days 21–23) indicates that a corpus luteum has been formed but cannot confirm that an egg was released. If ovulation does not occur, the corpus luteum does not form normally, and the rise in progesterone may be subnormal. As menstrual cycles vary in length, collection of a specimen for progesterone testing should be done approximately 1 week prior to the expected start of menses rather than a specific cycle day . Due to the wide fluctuations in progesterone concentrations, a progesterone concentration of >3 ng/mL is evidence of ovulation. Another indicator of ovulation is an increase in basal body temperature. Ovulation is associated with a rapid increase in body temperature by 0.5°F due to the increased progesterone concentration. Basal body temperature thermometers are inexpensive and simple to use, but they cannot be used to time intercourse because they indicate ovulation retrospectively. To prospectively indicate when ovulation should occur, serum or urine LH concentration can be measured. LH is detectable in the urine just after the serum LH surge and 24–36 h before ovulation. Serum concentrations of LH during the midcycle peak range from 21.9 to 56.6 IU/L. An alternative method to determine the LH surge is the use of home urine LH devices. These lateral flow qualitative immunoassays indicate the LH surge and become positive with urine LH concentrations generally >30 mIU/mL .
Ovarian reserve
Women in their mid to late 30s and early 40s with infertility constitute the largest portion of the total infertility population. These women also have a higher incidence of pregnancy loss. The decrease in fertility is due to a diminished ovarian reserve because of follicular depletion and decline in oocyte quality. As the number of follicles decreases with age, FSH concentrations in the follicular phase gradually increase. This increase in FSH is thought to be associated with the decreased production of inhibin B by the developing small antral follicles . Assessment of ovarian reserve via FSH and estradiol is performed by measuring both compounds on day 3 of the menstrual cycle. Day 3 FSH concentrations >5 IU/L are considered elevated and associated with poor reproductive outcome, as are basal estradiol concentrations >75 pg/mL .
Inhibin B has been proposed as a more direct measurement of ovarian reserve as it is directly produced by the developing follicles, and because it is produced by gonadal tissue, it is thought to be a more direct marker of ovarian reserve than pituitary hormones. Day 3 inhibin B concentrations are decreased in women with diminished ovarian reserve, and this inhibin B decrease can be detected prior to observation of an increase in FSH concentrations . Recently, anti-Müllerian hormone, a hormone produced predominantly by the large antral follicles of the ovary, has been examined as an indicator of ovarian reserve, and with a cutoff of 0.25 pg/mL, it provides higher sensitivity and specificity than age, FSH, estradiol, and antral follicle count . To date, studies examining the relationship between AMH and achieving successful pregnancy have not shown correlation between AMH concentrations and successful pregnancy .
AMH concentrations have however been found to be useful in the tailoring of ovarian stimulation protocols to reduce the risk of ovarian hyperstimulation syndrome (OHSS). OHSS is a potential complication of the use of exogenous gonadotropin-mediated stimulation of the ovaries for the purposes of oocyte retrieval as part of in vitro fertilization (IVF) treatment. This potentially life-threatening side effect of ovarian stimulation is due to the recruitment and maintenance of an unusually large number of follicles that evolve into luteal bodies following oocyte retrieval. The luteal bodies in addition to producing large concentrations of progesterone also produce vascular endothelial growth factor, which increases vascular permeability. OHSS is characterized by increased vascular permeability leading to fluid shifts from the vascular compartment to extravascular spaces. If sufficiently severe OHSS can cause edema, renal impairment, pericardial and pulmonary effusions, and ascites .
Structural factors
Evaluation of tubal patency should be done in a normal cycling woman to rule out structural abnormalities in the Fallopian tubes . Hysterosalpingography or hysterosalpingo-contrast ultrasonography can indicate whether there is a tubal blockage and give information about the shape of the uterine cavity. Hysterosalpingo-contrast ultrasonography has the added benefit of an ultrasound scan of the pelvis, which can identify fibroids or polycystic ovaries . Sonohysterography can identify abnormalities in the uterine cavity such as intrauterine adhesions, endometrial polyps, and submucosal fibroids .
Treatments
Patients dealing with infertility have many options to aid them when trying to conceive. Standard treatment for a woman with anovulation is ovulation induction with clomiphene citrate or gonadotropins followed by timed intercourse or intrauterine insemination. In the case of tubal sterility, diminished ovarian reserve, or the failure of standard treatments, IVF can be performed. The process of IVF includes ovarian stimulation, retrieval of the ova, fertilization, and then transfer of the fertilized ova into the uterus. A slightly different procedure called gamete intra-Fallopian transfer places the unfertilized ova and sperm into the Fallopian tube, whereas zygote intra-Fallopian transfer places a fertilized zygote into the Fallopian tube. Fertilization of the ova can also take place by direct intracytoplasmic sperm injection (ICSI) . Laboratory monitoring throughout all the assisted reproduction treatments discussed is necessary to determine not only the dose of therapy but also its duration and efficacy . For more in-depth information on this topic, the following reviews are recommended .
Male reproductive disorders
There are a number of abnormalities that can affect the male reproductive system throughout life ( Table 5.3 ). These include hypogonadotropic hypogonadism, hypergonadotropic hypogonadism, Klinefelter syndrome, defects in androgen action, erectile dysfunction, and gynecomastia. These abnormalities can also affect fertility, which will be discussed here in a separate infertility section.