ENDOCRINE ASPECTS OF HYPERTENSION
Dalila B. Corry
Michael L. Tuck
SPECTRUM OF HYPERTENSION
Hypertension is an extremely common disorder, affecting 15% to 20% of the population. More than 95% of hypertension is of unknown etiology, termed primary or essential hypertension. There are several causes of endocrine hypertension (Table 82-1), with the most common forms being renal artery stenosis, primary aldosteronism, and pheochromocytoma. In the Hypertension Detection Follow-Up Program, 0.18% of 15,000 participants had secondary hypertension.1 Because endocrine hypertension is rare, it is recommended by the Joint National Committee on Hypertension VI (JNC VI) that the new-onset hypertensive patient not have extensive testing for endocrine hypertension.2 Thus, only if the history and physical examination suggest endocrine hypertension is it necessary to perform screening tests. Pheochromocytoma can be detected with high sensitivity and specificity using urine and plasma catecholamine measurements. Primary aldosteronism is suspected by low serum potassium and plasma renin activity (PRA); however, as screening tests, these measurements are not very sensitive or specific. The screening tests for renovascular hypertension are improving, but most testing procedures are either invasive or indirect and can be costly.
Secondary hypertension should be suspected in severe and resistant hypertension, new-onset hypertension in the young (<20 years) or the older patient (>55 years), hypertension with spontaneous hypokalemia, and episodic hypertension with sweating, tachycardia, and headaches. Important physical findings include the detection of an abdominal bruit (suggestive of renovascular hypertension); a radial-femoral pulse delay (suggestive of aortic coarctation); or central obesity, striae, and bruising (suggestive of Cushing syndrome). Other chapters deal in depth with aldosteronism, Cushing syndrome, congenital adrenal hyperplasia, and pheochromocytoma (see Chap. 75, Chap. 77, Chap. 80, and Chap. 86). This chapter deals with selected topics.
RENOVASCULAR HYPERTENSION
Renovascular hypertension is the most common cause of secondary hypertension (1% of cases of mild to moderate hypertension and up to 10% to 40% of subjects with acute, severe, or refractory hypertension).3 There is still much debate over the method and extent of evaluation in such patients.3,4,5,6,7 and 8
Clinically, diffuse atherosclerosis with severe hypertension can be associated with a high incidence.9,10 Acute elevation of serum creatinine is another presentation, either spontaneously or after initiation of an angiotensin-converting enzyme (ACE) inhibitor.5 Asymmetric renal size is a clue, as are recurrent episodes of acute (“flash”) pulmonary edema and unexplained congestive heart failure.11
Hypertension results from obstructive lesions of the main renal artery or its segmental branches, but it takes ≥70% stenosis to lead to hypoperfusion, reduced renal blood flow, and ischemia. Thus, the primary anatomic stenotic lesion may or may not be related to the blood pressure (BP), as it takes significant stenosis to activate the renin-angiotensin system, the main mediator of hypertension in renovascular hypertension.
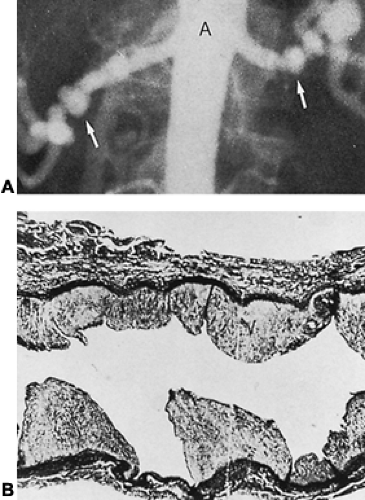
Most renovascular hypertension results from either atherosclerotic plaques or fibromuscular dysplasia. Of 2442 hypertensive patients included in the Renovascular Cooperative Study,3 63% had atherosclerotic lesions and 32% had fibromuscular dysplasia. Fibromuscular dysplasia occurs mostly in children and young adults, especially in women, whereas atherosclerotic plaques are seen in men over 45 who have a history of cigarette smoking. The most common form of fibrous renal artery disease is medial fibroplasia; often it is bilateral (Fig. 82-1).
The syndrome of renovascular hypertension requires a broad definition (i.e., any secondary elevation of BP by any condition that interferes with the arterial circulation to kidney tissue).12,13 Trauma and subcapsular hematoma can exert pressure on the kidney, causing renal ischemia without stenosis of the renal arteries. Other causes include renal transplantation, necrotizing vasculitis, intravenous drug abuse, malignant hypertension, renal artery emboli, coarctation or dissection of the aorta, and renal artery aneurysms.13
PATHOGENESIS
The two main types of renovascular hypertension are unilateral and bilateral renal artery stenosis. The renin-angiotensin system is
the major hormone system in the pathogenesis of renovascular hypertension, with the critical initiating event being reduced renal perfusion pressure14,15,16,17 and 18 (see Chap. 79 and Chap. 183). There is increased renin and angiotensin II (A-II) production by the stenotic kidney, although PRA levels often are normal to only moderately elevated, reflecting renin suppression in the non-stenotic kidney. In experimental one-kidney, one-clip renovascular hypertension, the renin levels increase for 6 to 10 days after renal artery clipping and then decline, yet the hypertension persists. The long-term increase in BP is related to increases in vascular resistance; the early enhancement of the renin-angiotensin system triggers other factors, sustaining the hypertension. (Volume expansion may play a critical role in long-term BP elevations.) In contrast, in the two-kidney, one-clip model of renovascular hypertension, an elevated PRA persists throughout the chronic maintenance phase of hypertension.
the major hormone system in the pathogenesis of renovascular hypertension, with the critical initiating event being reduced renal perfusion pressure14,15,16,17 and 18 (see Chap. 79 and Chap. 183). There is increased renin and angiotensin II (A-II) production by the stenotic kidney, although PRA levels often are normal to only moderately elevated, reflecting renin suppression in the non-stenotic kidney. In experimental one-kidney, one-clip renovascular hypertension, the renin levels increase for 6 to 10 days after renal artery clipping and then decline, yet the hypertension persists. The long-term increase in BP is related to increases in vascular resistance; the early enhancement of the renin-angiotensin system triggers other factors, sustaining the hypertension. (Volume expansion may play a critical role in long-term BP elevations.) In contrast, in the two-kidney, one-clip model of renovascular hypertension, an elevated PRA persists throughout the chronic maintenance phase of hypertension.
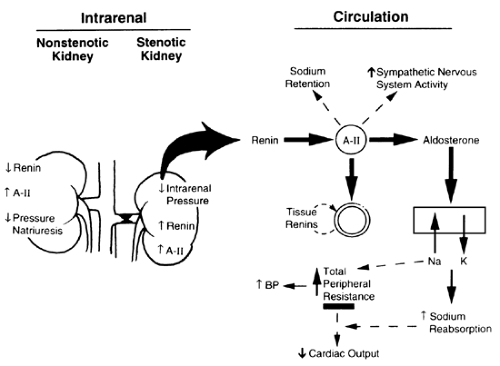
In unilateral renal artery stenosis, the uninvolved kidney should protect against fluid and electrolyte imbalance and hypertension; the rise in BP should increase sodium excretion in the nonstenotic kidney. However, the nonstenotic kidney does not adapt appropriately; there is a paradoxical rise in local A-II, despite a suppressed PRA15,16 (Fig. 82-2). This discordant response leads to mediation by A-II of vasoconstriction and sodium retention with a blunted pressure natriuresis response curve in the nonstenotic kidney.
In bilateral renovascular hypertension, the hormonal, neural, and volume responses are different from those in unilateral disease. There is an initial rise in the renin-angiotensin system, but with time, there is normalization of the renin-angiotensin system activity, and hypertension in the steady-state condition becomes more dependent on volume-mediated factors.
Other amplifying factors contribute to the maintenance of hypertension in renovascular hypertension; there may be a neurogenic phase, as enhanced sympathetic nervous system activity is found in the early stages.19 Levels of several vasoactive eicosanoids are high in animals with renovascular hypertension.14,16 A-II–induced activation of the lipoxygenase pathway of arachidonic acid has pressor functions and has been implicated in the sustained phase. Lipoxygenase inhibitors prevent the development of experimental renovascular hypertension.19 A-II in the kidney is taken up and incorporated by renal cells through the A-II–AT1 receptor16; this uptake might serve to prolong A-II action. Circulating levels of aldosterone are normal to high in renovascular disease, depending on the levels of renin. In marked renovascular hypertension, renin and aldosterone levels are high, and hypokalemia is found.20
CLINICAL CLUES TO SCREEN FOR RENOVASCULAR HYPERTENSION
Abrupt onset of severe hypertension in a young (younger than 20 years) or older individual (older than 55 years).
Prepubertal hypertension.
Hypertension that is refractory to triple-drug therapy.
Malignant or accelerated hypertension associated with grade III to IV retinopathy.
Sudden flank pain due to renal embolic infarction.
Unexplained azotemia.
Detection of an upper flank or abdominal bruit that radiates to the flanks. (The bruit may be systolic, systolic-diastolic, or continuous, and is high pitched and difficult to discern.) Bruit in a hypertensive young woman suggests fibromuscular disease.
Hypertension associated with diffuse atherosclerotic vascular disease, especially in a smoker.
Unexplained deterioration in renal function with the use of an ACE inhibitor.
Paradoxical worsening of hypertension with a diuretic.
Spontaneous hypokalemia.
Recurrent flash pulmonary edema.
LABORATORY EVALUATION
Routine Laboratory Tests.
Measurements of electrolytes, renal function tests, and urinalysis often give normal results in renovascular hypertension.2 Hypokalemia is found in those cases in which secondary hyperaldosteronism is found. In unilateral stenosis of the renal artery, an impairment of renal function occurs infrequently, whereas in bilateral renal artery stenosis, serum creatinine and urea nitrogen may be increased. In embolic renovascular disease, hematuria may be detected.
SCREENING TESTS
Intravenous Pyelography.
The findings on rapid-sequence pyelography that have the greatest significance are a unilateral decrease in renal size (>1.5 cm disparity in pole-to-pole diameter) and a unilateral delay in the appearance of contrast medium in the 1- to 5-minute exposures.3 False-negative results often occur in cases of renovascular hypertension because of bilateral renal artery disease.3,21 The hypertensive urogram has low sensitivity (a negative test does not exclude renovascular hypertension) and specificity and, importantly, has the risk of nephrotoxicity because of contrast agents.
Plasma Renin Activity.
Although hypoperfusion in the stenotic kidney activates the renin-angiotensin system, renin secretion in the nonstenotic kidney is often normal or low, so that the baseline or unstimulated circulating PRA levels are normal in most cases. Thus, when measured in peripheral venous samples, PRA is normal in 20% to 50% of cases of documented renovascular hypertension.7,13,20 The interpretation is confounded by the fact that 16% to 20% of subjects with essential hypertension have PRA values that are moderately elevated.
However, in renovascular hypertension there are exaggerated PRA responses to procedures that stimulate the renin-angiotensin system, such as upright posture, sodium restriction, and the administration of angiotensin-converting enzyme inhibitors (ACEI).22 ACEI interrupt the conversion of A-I to A-II; the negative feedback effect of A-II on renin release is diminished, resulting in an increase in secretion. The administration of the ACEI captopril to patients with renovascular hypertension produces a marked increase in PRA compared to responses in other forms of hypertension.20,22,23 and 24 In the usual test procedure, captopril, 25 to 50 mg, is given orally, and PRA is obtained after 1 hour. The test has 75% to 100% sensitivity and 60% to 90% specificity.22,24 Furthermore, it is safe and inexpensive, and can be done on an outpatient basis or simultaneously with captopril renography. The utility of the test is hampered by the need for strict standardization, the need to stop antihypertensive medications, its reduced accuracy in renal failure, and low sensitivity and poor predictive value compared to a renogram.
Renal vein renin levels, sampled by percutaneous catheterization, determine the functional significance of the stenotic lesion and predict the BP response to therapy.3 Renin production is increased in the stenotic kidney and suppressed in the contralateral kidney; asymmetry of renin production yields a renal vein renin ratio of ≥1.5:1 in renovascular hypertension. This procedure has predictive value for surgical curability, but use has declined because it is invasive, and up to 60% of patients do not lateralize but are nevertheless improved by surgery.25
Radionuclide Renography.
The renogram relies on a disparity of renal function and of perfusion between the stenotic and nonstenotic kidneys. Isotopic compounds such as technetium-99m–diethylenetetraminepentaacetic acid (DTPA) accurately measure glomerular filtration rate, and compounds such as 99mTc-mercapto acetyl triglycine (99mTc-MAG3) measure both glomerular filtration rate and tubular secretion and are good in renal insufficiency.7 The addition of computer quantitation has improved their accuracy.3,4,5,6 and 7,13,26 Patients with renovascular hypertension show decreased uptake, delayed peak values, and prolonged excretion of these agents. Because of the low-risk and noninvasive nature of renography, it has many advantages. However, renography used alone has a high incidence of false-negative and false-positive results.
Captopril administration during renography has improved its sensitivity and specificity.5,6 and 7,22,24,26,27 Captopril (25–50 mg), which is given 1 hour before the isotope injection, reduces A-II–mediated vasoconstriction in the efferent arterioles and lowers glomerular pressure and filtration rate. In the stenotic kidney, captopril reduces renal perfusion more than in the nonstenotic kidney, thereby revealing the asymmetric reduction in renal function in renovascular hypertension. Scintiphotographs and time-activity curves are analyzed to assess renal perfusion, function, and size. In high-risk populations, sensitivity and specificity exceed 90% for high-grade stenosis. The predictive value of the ACE-inhibitor renogram is less in low-risk populations and in bilateral disease.
Duplex Doppler Ultrasonography.
Ultrasonography has been improved by the use of low-frequency transducers (B-mode imaging) for better visualization of the renal arteries, and Doppler for the measurement of differential blood flow velocities.28,29,30 and 31 It has the advantage of determining both the anatomic and the functional significance of a stenotic lesion. A renal-aortic flow velocity ratio of ≥3.5 suggests renovascular hypertension. Duplex Doppler ultrasonography can detect unilateral and bilateral disease, can detect recurrent stenosis in previously treated patients, and can be enhanced with ACE inhibition30 and color coding.31 It has a 99% positive predictive value and a 97% negative predictive value.29 (Disadvantages: time-consuming and highly operator-dependent.)
Magnetic Resonance Angiography.
Magnetic resonance (MR) angiography is promising and noninvasive, and may become the method of choice.32,33,34,35 and 36 In comparison with arteriography, MR angiography showed 100% sensitivity and 96% specificity for finding stenosis of the main renal arteries.32,33,34,35 and 36 A sensitivity of 100% and a specificity of 71% were found for lesions of the proximal renal arteries with 50% to 75% stenosis.34 Significant advances in techniques include breath-holding MR angiography and paramagnetic contrast material. Thus, the procedure may be able to visualize accessory arteries.34 Phase-contrast MR angiography may enable determination of the hemodynamic significance of a stenotic lesion.35 The procedure cannot be used in individuals with metallic implants such as a pacemaker or a clip for an aneurysm.
Spiral (Helical) Computed Tomographic Scan and Computed Tomographic Angiography.
This offers great promise as the best noninvasive screening test.37 In a comparison of spiral computed tomography (CT) to arteriography in subjects investigated for renovascular hypertension, the sensitivity was 98% and the specificity 94%.28 However, renal insufficiency reduces the accuracy of this procedure due to diminishing renal
blood flow. It identifies lesions of the main renal arteries but has more difficulty in finding branch lesions. Although it is minimally invasive, the risk of radiocontrast-induced nephrotoxicity still exists.
blood flow. It identifies lesions of the main renal arteries but has more difficulty in finding branch lesions. Although it is minimally invasive, the risk of radiocontrast-induced nephrotoxicity still exists.
Angiography.
Despite the increase in types of procedures and technical advances in detecting renovascular hypertension, renal angiography by the percutaneous, transfemoral route remains the “gold standard” for detecting renal artery stenosis, as well as its location and pathology. Atherosclerotic lesions most often involve the proximal segment of the renal artery and have a circular configuration. By contrast, fibromuscular disease is in the more distal portions of the renal artery and is either localized or diffuse and bilateral. Angiography is costly and invasive and has the risk of nephrotoxicity. Because angiography does not always predict the functional significance of renal artery stenosis or its treatment outcome, many centers apply simultaneous angioplasty, with the BP response to percutaneous renal angioplasty or stenting serving as the indicator of functional significance.
Digital subtraction angiography avoids some of the complications of percutaneous catheterization, because computer enhancement of the renal area allows the injection of smaller amounts of contrast material.3 Injection has been in a peripheral vein, but the specificity and sensitivity of the procedure are both 90% or less when compared to arterial procedures. Because 150 to 200 mL of dye is injected, there is still risk for nephrotoxicity. An intraaortic injection site provides better visualization. The procedure can be performed safely in azotemic patients, and it has been useful in hypertension after renal transplantation.4
Thus, the renal arteriogram best establishes the presence of renal artery stenosis but does not distinguish functional from nonfunctional lesions. Tests such as the intravenous pyelogram and radionucleotide renogram lack sensitivity for detecting renovascular hypertension and, if used alone, cannot consistently diagnose renovascular hypertension. The newer tests (e.g., captopril renography, MR angiography, duplex ultra-sonography, and spiral CT) show great promise.
TREATMENT OF RENOVASCULAR HYPERTENSION
The goals of therapy of renovascular hypertension are the control of BP and the preservation of renal function. The three therapies for treatment of renovascular hypertension are medical, percutaneous transluminal renal angioplasty, and surgery. Each approach has advantages and disadvantages, and the selection also depends on cause, severity, and age. Few trials have compared the three therapies. Often no single test can help in this decision, and experience and clinical judgment are paramount. There are several differences in the approach to unilateral versus bilateral renal artery stenosis.
Unilateral Renal Artery Stenosis
SURGERY.
Often, surgery is reserved for patients for whom hypertension cannot be controlled on medical therapy or for those who fail angioplasty and in whom renal function is deteriorating. However, some believe that surgery should be considered in patients <50 to 60 years of age, as the outcome is more definitive and spares the chronic use of medication.38,39,40 and 41 Surgery is generally more effective than angioplasty, especially in patients with atherosclerosis. One indication for surgical revascularization is preservation of renal function, based on the emerging evidence of deteriorating renal function in atherosclerotic ischemic renal disease.41,42 and 43 Revascularization procedures include bypass using natural or synthetic arteries; natural arteries provide a better outcome.38,39,40 and 41 The surgical cure rate for hypertension due to unilateral atherosclerotic lesions may be up to 95%, especially if the disease is of short duration. Less favorable results are seen in older subjects with long-standing hypertension, probably due to either underlying essential hypertension or the onset of intrarenal disease. Mortality from surgery is ˜2.5% in experienced centers; it is higher in older patients, especially those with diffuse atherosclerosis and heart failure.40
PERCUTANEOUS TRANSLUMINAL ANGIOPLASTY.
The technical success and cure rate for percutaneous transluminal angioplasty are rapidly improving; this procedure and stenting are now treatments of choice.44,45,46,47,48,49 and 50 Patients with fibromuscular dysplasia have the best outcome (<10% restenosis). Best results are obtained in lesions of the main renal artery that are only partly occluded.44,45,46 and 47 In atherosclerotic lesions the results are not as good. Cure rates of only 10% to 20% are recorded; ˜60% will improve but not be cured, and 15% incur no benefit. A failure can be predicted in the first 48 hours if BP fails to fall.46 Technical complications include renal artery thrombosis and perforation. The main overall advantage of percutaneous transluminal angioplasty may be the ability to decrease the number of medications needed to control BP without the risks of surgery.46,47 In transplant renal artery stenosis, angioplasty is first-line therapy, with a cure rate of 70% at 5 years; surgery is less successful because of extensive scar tissue.3 Ostial stenoses are less likely to respond to angioplasty (recurrence rate as high as 47% for atherosclerotic lesions). Here, the outcome of angioplasty is better with intravascular stents.48,49 and 50 Stents are placed most often after unsuccessful angioplasty and offset the effect of elastic recoil (success rate from 65% to 70%; restenosis in only 13%).48,49
MEDICAL THERAPY.
In high-risk individuals with coexisting carotid or coronary artery disease in which surgical or angiographic risk is high, medical therapy is often the sole modality.51,52 and 53 However, the natural course of renovascular hypertension, especially due to atherosclerotic disease, shows that many stenotic lesions progress over time and that renal damage can occur in untreated lesions.52 Thus, it remains uncertain whether drug therapy—by only controlling BP—affects the progression of renovascular hypertension.
Because renovascular hypertension is a renin-dependent form of hypertension, ACEI and A-II–receptor blockers should show selective efficacy.51 In unilateral renovascular hypertension, ACEI may be as effective as monotherapy.51 However, ACE inhibitors can produce rapid loss of renal function especially in bilateral renovascular hypertension or in renovascular hypertension with a solitary kidney, owing to the almost complete dependency of renal function on A-II.51 Usually, the use of ACEI in unilateral renovascular hypertension does not permanently reduce glomerular filtration and is safe and effective.51 Some clinicians monitor serum creatinine, renal size, and periodic ultrasonography during such therapy.
Calcium antagonists are effective antihypertensive agents in renovascular hypertension and may induce less renal impairment than ACE inhibitors.3 However, in critical, high-grade stenosis, any agent that lowers BP excessively can impair renal function. A-II–receptor blockers act by directly blocking the AT1 receptor without affecting other peptides (e.g., bradykinin) as do the ACEI.
Bilateral Renal Artery Stenosis.
Bilateral renal artery stenosis or unilateral stenosis in a solitary kidney usually involves more acute, severe, and difficult-to-treat hypertension, accompanied often by renal insufficiency. In bilateral renovascular hypertension, treatment should be guided toward lowering BP and preserving renal function. Patients who have bilateral disease with complete stenosis of one renal artery are at the greatest risk. Doppler ultrasound follow-up in bilateral renovascular hypertension shows that by 1 year, there often is a decrease in kidney size.54 To preserve renal function, the use of surgery or angioplasty should be considered in bilateral disease.
Percutaneous transluminal angioplasty is generally less successful in bilateral renovascular hypertension and is associated with more complications (atheroemboli).42,55 It is limited to people who are at high surgical risk or those who cannot be controlled by medication. Intravascular stents may expand the success of angioplasty in this disease.
Medical therapy in bilateral disease is best handled with ACEI or a calcium-channel blocker with the addition of a diuretic based on the volume expansion and volume-dependent
hypertension.51 One can almost expect a hemodynamic decline in glomerular filtration rate following ACEI in bilateral renovascular hypertension, so careful monitoring is essential.
hypertension.51 One can almost expect a hemodynamic decline in glomerular filtration rate following ACEI in bilateral renovascular hypertension, so careful monitoring is essential.
If possible, the surgical correction of bilateral renal artery stenosis should be pursued, because BP and renal function can improve after revascularization.40,56 Optimal therapy for bilateral disease has not been established: Surgery is the treatment of choice in the young with bilateral severe stenosis. In older, high-risk subjects with atherosclerosis, medical therapy with either an ACEI or a calcium-channel blocker is indicated.
RENIN-PRODUCING TUMORS
Renin-producing tumors, a rare form of endocrine hypertension that occurs in young persons, manifest severe hypertension and hypokalemia.56,57 The PRA is among the highest recorded in hypertensive syndromes and can exceed 50 ng/mL/h.56 The hypokalemia, often <2.0 mEq/L, is due to intense secondary hyperaldosteronism. The combined high PRA and aldosterone levels distinguish this disorder from primary aldosteronism. Two categories of renin-producing tumors cause this syndrome: renal juxtaglomerular cell tumors and a variety of extrarenal tumors, such as Wilms and ovarian tumors.56,57 The renin originating from tumor production resembles that found in normal kidneys.58 Extrarenal renin-secreting tumors have higher concentrations of prorenin than do tumors of renal origin.57,59 Most cases of extrarenal renin-secreting tumors occur in women, and many are located in the reproductive tract.59 In these patients, the high prorenin level serves as a marker for the tumor. One presentation is the hyponatremic hypertensive syndrome and massive proteinuria seen in patients with renin-producing leiomyosarcomas.60 The ACEI captopril is effective in the control of BP due to renin tumors.61
In renal renin-secreting tumors, renal vein PRA measurements can localize the tumor. They are small, so that radiologic visualization, including urography, magnetic resonance imaging (MRI), ultrasonography, CT, and angiography may sometimes be unsuccessful57,62; even exploratory surgery may fail to find the lesion. In these patients, nephrectomy or selective tumor resection is curative, and ACEI are the medical treatment of choice.28 An A-II–receptor blocker may be equally effective or superior by also blocking the antigensin type 2 (AT2) receptor.63
MINERALOCORTICOID HYPERTENSION
Several mineralocorticoids, such as aldosterone and deoxycorticosterone (DOC), produce hypertensive syndromes.64,65 and 66 For more details of aldosterone function see review.66a Primary aldosteronism is the best example of mineralocorticoid hypertension. The mechanisms underlying hypertension include sodium retention, extracellular fluid expansion, high cardiac output, increased sympathetic nervous system activity, and structural changes in blood vessels. Hyperaldosteronism is reviewed elsewhere (see Chap. 80), as is congenital adrenal hyperplasia (see Chap. 77).
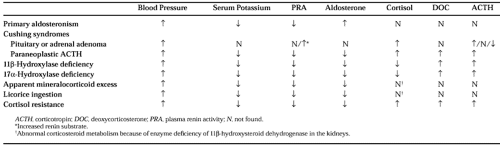
Table 82-2 summarizes the levels of BP, serum potassium, PRA, aldosterone, cortisol, DOC, and corticotropin (ACTH) in several glucocorticoid and mineralocorticoid disorders associated with hypertension. Glucocorticoid resistance results in a mineralocorticoid form of hypertension accompanied by hypokalemia and suppressed renin and aldosterone (see Table 82-2). High levels of plasma cortisol and a paucity of stigmata of Cushing syndrome suggest the diagnosis.67 The insensitivity to glucocorticoids is caused by inherited glucocorticoid receptor mutations.67 An insensitivity to cortisol at the hypothalamic-pituitary feedback for ACTH increases ACTH production, which, in turn, stimulates DOC and corticosterone formation; the increased mineralocorticoid activity then causes volume expansion, hypertension, and the suppression of renin and aldosterone. An adrenal androgen excess can also be found, resulting in hirsutism in women and precocious pseudopuberty in children. The demonstration of a functionally abnormal glucocorticoid receptor in mononuclear cells confirms the diagnosis.67 Mutations occur in the glucocorticoid receptor–ligand-binding domain and at a splice site.67 High doses of dexa-methasone reduce the ACTH, and thereby correct the BP and serum potassium abnormalities. A form of cortisol resistance has also been described in the acquired immunodeficiency syndrome.67,68
HYPERTENSION IN OTHER ENDOCRINE DISORDERS
CUSHING SYNDROME
Hypertension is common in endogenous Cushing syndrome (up to 80%) but occurs less frequently with exogenous glucocorticoid therapy.69 The cardiovascular mortality and morbidity are much higher in Cushing syndrome,70 attributed to the effects of cortisol on BP, on atherosclerosis, and on the heart. The hypertension is thought to directly result from cortisol excess through at least three mechanisms.71 Glucocorticoid excess increases the hepatic synthesis of angiotensinogen, the substrate for the production of the vasoconstrictor A-II. This mechanism might suggest a renin-dependent form of hypertension, yet renin levels in Cushing syndrome often are normal to decreased. Glucocorticoid administration to normal subjects will enhance vascular reactivity to pressor hormones such as phenylephrine; indeed, many attribute its hypertensive action to this mechanism. Although cortisol in excess binds to mineralocorticoid receptors, most cases of Cushing syndrome resulting from pituitary or adrenal adenoma do not have strong evidence for mineralocorticoid excess such as hypokalemia and suppressed renin.
A more pronounced mineralocorticoid effect is seen in Cushing syndrome due to paraneoplastic ACTH production. In these
disorders, the high levels of cortisol and DOC, but not aldosterone, cause a mineralocorticoid effect, leading to hypokalemia and hypertension71 (see Chap. 219). High circulating ACTH levels in these ectopic syndromes preferentially stimulate adrenal secretion of DOC over aldosterone; the excess DOC causes volume expansion and the suppression of renin and aldosterone. One should be alerted to this scenario in an established or suspected cancer patient—with unexplained hypokalemia and metabolic alkalosis—who may or may not have hypertension.
disorders, the high levels of cortisol and DOC, but not aldosterone, cause a mineralocorticoid effect, leading to hypokalemia and hypertension71 (see Chap. 219). High circulating ACTH levels in these ectopic syndromes preferentially stimulate adrenal secretion of DOC over aldosterone; the excess DOC causes volume expansion and the suppression of renin and aldosterone. One should be alerted to this scenario in an established or suspected cancer patient—with unexplained hypokalemia and metabolic alkalosis—who may or may not have hypertension.
Another mechanism for hypertension in paraneoplastic ACTH syndromes is one in which the renal form of the enzyme 11β-hydroxysteroid dehydrogenase (11β-HSD) is deficient or overwhelmed by the high circulating cortisol levels.72 This defect leads to the accumulation of cortisol in the kidneys, where it binds in excess to the mineralocorticoid receptor. Diagnosis is made by demonstrating a high ratio of the urine metabolites of cortisol/cortisone. These findings are similar to those seen in the genetic or acquired (licorice) forms of apparent mineralocorticoid excess.66 In some cases of adrenal carcinoma, there are partial enzyme blocks leading to the accumulation of steroid precursors (e.g., compound DOC), which can have mineralocorticoid effects.
DIABETES MELLITUS AND HYPERTENSION
The frequency of hypertension in diabetes mellitus is high, reaching an incidence rate of about 50%.73,74 Hypertension in the diabetic greatly enhances the risk of macrovascular atherosclerotic complications and the microvascular complications of nephropathy and retinopathy. It is also known that lowering BP slows the progression of diabetic nephropathy75 and diabetic retinopathy.76
In type 1 diabetes mellitus, hypertension appears after several years and is seen almost uniquely in those 40% of type 1 diabetics who will develop diabetic nephropathy. The BP begins to rise at about 3 years after the onset of microalbuminuria, and hypertension is eventually seen in 85% of them. Thus, renal mechanisms explain most of the pathophysiology of hypertension in type 1 diabetes mellitus. In type 2 diabetics, 40% to 70% develop hypertension. Here, the hypertension is due to aging, obesity, insulin resistance, hyperinsulinemia, atherosclerosis, and renal disease; it may be present at the time of diagnosis or even have preceded the onset of diabetes mellitus. However, a significant number of type 2 diabetics have microalbuminuria, suggesting that renal disease can be a contributor. Nevertheless, obesity probably is the major cause of their hypertension; genetic factors also contribute, as there is an association between the A2 allele of the glycogen synthase gene and insulin resistance.77
Unique mechanisms for the cause of hypertension in diabetes mellitus relate to the metabolic abnormalities; both type 1 and type 2 diabetics have increased vascular reactivity and sodium retention.78,79 Norepinephrine, A-II, exercise, and mental stress in diabetic subjects lead to exaggerated BP responses.78,79 Sodium retention and volume expansion in the diabetic are due to increased reabsorption through tubular glucose-sodium cotransport80; this causes a higher incidence of salt-sensitive hypertension.81
High levels of insulin in diabetes contribute to the hypertension. In type 2 diabetics, insulin resistance causes hyperinsulinemia; also, insulin excess may occur in type 1 diabetes due to exogenous therapy.74 Insulin increases sodium reabsorption and sympathetic nervous system activity and has direct effects on vascular reactivity.73,74 After starting insulin in type 2 diabetics, there is an increase in mean values of BP from 132/81 to 148/89 mm Hg.82
In human diabetes, as well as in experimental models, values for plasma renin activity often are reduced or completely suppressed,83 whereas in certain tissues (e.g., the kidney) the expression of renin and its products is elevated.84 Individuals with long-standing diabetes and renal impairment can have the syndrome of hyporeninemic hypoaldosteronism, which is detected by finding low PRA and aldosterone levels and hyperkalemia. The low PRA in diabetic patients is due to diabetic nephropathy, destruction of the juxtaglomerular apparatus, defective conversion of prorenin to active renin, chronic volume expansion, and decreased neural control of renin release.83,84 The efficacy of ACEI in treatment of hypertension and target-organ protection in diabetes mellitus occurs despite low circulating levels of renin and is thought to be due to effects on the tissue renin-angiotensin system84 and increases in bradykinin.85 Endothelial dysfunction, which is common in diabetes, may further contribute to the hypertension86; in type 1 diabetics, ACEI therapy improves endothelial function.87
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree