Fig. 1
Main clinical manifestations after remission of Cushing’s syndrome
This chapter addresses current information on the main long-term/persistent effects of prior Cushing’s disease/glucocorticoid exposure.
Cardiovascular and Metabolic Comorbidities
Hypercortisolism enhances cardiovascular risk factors such as glucose intolerance, central obesity, hypertension, and dyslipidemia. All are linked to an increased incidence of atherosclerosis and coronary disease, and impact on morbidity, cardiovascular disease being the leading cause of death in patients with Cushing’s syndrome (CS). However, this cardiovascular risk profile is not completely explained by conventional cardiovascular risk factors; other still inadequately defined disease-specific factors, partially related to the hypercoagulable and inflammatory state with an unfavorable adipokine profile, have also been observed [1]. Although most of the risk factors improve, cardiovascular risk is clearly increased in CS patients even years after remission (Fig. 2) [2].
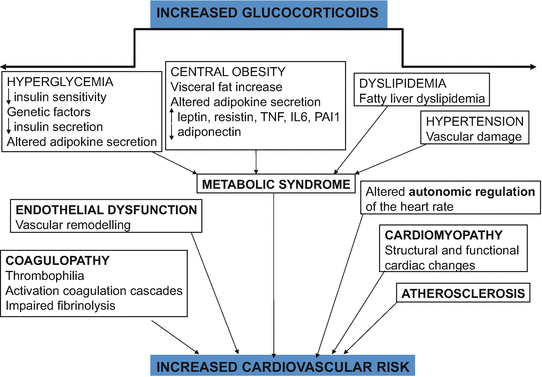

Fig. 2
Cardiovascular risk in Cushing’s syndrome
Glucose Metabolism
Glucose metabolism abnormalities are common in CS; in fact diabetes is one of the most common metabolic complications of CS . The prevalence of these abnormalities varies depending on the series and the etiology of CS (higher in ectopic CS compared to pituitary or adrenal adenomas) [3]. Prevalence of impaired glucose tolerance is estimated around 21–64 % and of overt diabetes mellitus around 20–47 %; the latter decreases by 40 % after biochemical control of hypercortisolism, but is still higher than body mass index (BMI)-matched controls after 5 years of cortisol normalization (33 vs. 7 %) [2, 4]. It is worth noting that this prevalence may be underestimated, since not all patients underwent an oral glucose tolerance test, required to diagnose impaired glucose tolerance (IGT) when fasting glucose is normal.
GCs affect glucose homeostasis through the induction of gluconeogenesis, disruption of insulin receptor signaling, and reducing insulin sensitivity in liver and skeletal muscle [5]. Although hypercortisolism is involved in this higher prevalence of glucose metabolism abnormalities, it seems that age, genetic predisposition, lifestyle, and degree of exposure to hypercortisolism may all contribute to these deleterious effects [6]. Insulin resistance persists after biochemical remission of hypercortisolism, independently of body weight, suggesting that reduction in insulin sensitivity is not due to obesity but to hypercortisolism per se. Although insulin levels in patients in remission were observed to be lower than in active disease, both groups of CS patients had higher levels of insulin compared to healthy controls [7].
Obesity, Central Adiposity, and Chronic Inflammatory State
Chronic hypercortisolism determines a redistribution of body fat leading to increased abdominal fat and reduced peripheral subcutaneous adipose depots, with the related metabolic consequences.
Several studies have observed a higher body mass index and waist/hip ratio in CS patients compared to an age- and sex-matched controls. Persistently increased abdominal circumference was seen in CS patients (irrespective of the cause) 1 year after hormonal remission [8]. In a recent published study evaluating cardiovascular risk factors after remission of hypercortisolism , the authors observed that all the risk factors returned to a level comparable to the control subjects, except for obesity and triglyceridemia (related directly to central obesity) [1]. When comparing body composition before surgery and in remission (mean of 20 months after surgery) using whole body magnetic resonance imaging, although an important part of the fat depots had decreased and reverted fat to a distribution more consistent with favorable cardiovascular risk, most patients with Cushing’s disease (CD) in remission continued to have overweight, obese, and had persistence of cardiovascular risk [9]. A case–control study showed that patients with CS after a mean of 11 years in remission continued to have greater total fat and central obesity as compared to age- and sex-matched controls [7]. In the same line, in a group of 50 women with CS in remission (median time 13 years), abdominal fat mass was increased compared to matched controls. The authors also observed that increased abdominal obesity was associated to ongoing GC replacement therapy, as well to polymorphism rs1045642 in a ABCB1 gene, related to GC sensitivity [10]. Although correction of hypercortisolism is generally associated with a reduction of visceral and subcutaneous fat mass, body cell mass loss does not recover after remission, indicating true protein loss in these patients [11].
Moreover, it seems that the effects of exogenous hypercortisolismon body composition is different from those seen in endogenous CS, where the increase in total body fat and trunk fat is higher [12]. Recently, glucocorticoid-induced obesity has been evaluated among different diagnostic groups of CS. Interestingly, patients with primary pigmented nodular adrenocortical disease who presented a PRKAR1A gene mutation (increased cAMP-dependent protein kinase levels) were less obese than other patients with CS [13, 14].
Altered Adipokine Secretion
This increased central obesity and visceral adiposity characteristic of CS induces impaired adipokine production. The persistence of central adiposity and an unfavorable adipokine profile may link metabolic alterations and cardiovascular morbidity in CS after biochemical remission. Some adipokines may contribute to the pathogenesis of vascular, metabolic and inflammatory complications such as endothelial damage, high blood pressure, impaired bone remodeling, atherosclerosis, and low grade inflammation [15].
Increased levels of leptin, resistin, and proinflammatory cytokines such as tumor necrosis factor alpha (TNF-alfa) and interleukin-6 observed both in active CS and even years after biochemical remission are associated with greater cardiovascular risk [7, 15, 16]. These and other adipokines and humoral factors may stimulate circulating cortisol levels (activating 11ß-hydroxysteroid dehydrogenase type 1 11ß-HSD1), contributing to the typical characteristics of metabolic syndrome and visceral obesity in CS [17].
Leptin , an anorexigenic hormone, in general is elevated in active CS. It decreases after correction of hypercortisolism , depending on the timed evaluation and changes in body fat [15]. Leptin elevation persists 10 days after surgery for CD despite a drop in cortisol levels, suggesting that factors other than cortisol, such as persistently abnormal fat distribution, play a role in leptin hypersecretion [18]. In long-term remission of hypercortisolism , leptin gradually decreases in parallel to a decrease in BMI, fat mass, and insulin levels [9]. Also, a decrease in leptin concentrations, 9 months after curative surgery in CD patients, was observed, similar to findings in obese patients following bariatric surgery [16, 19].
On the other hand, adiponectin (an adipokine with antiatherogenic and anti-inflammatory properties) is decreased in patients with active and cured CS after 11 years of biochemical control compared to controls; however, the differences were no longer significant when patients were stratified based on their estrogen status [7]. Nonobese CS patients had lower adiponectin concentrations compared to non-obese controls, but this difference was not present when comparing obese CS patients and obese controls. This suggests that obesity is crucial when considering adiponectin levels in CS patients [20]. Another peptide with anti-inflammatory, as well as antifibrotic effects (although not an adipokine), is ghrelin; its levelsy have been found to be higher 24 months after successful surgical correction of hypercortisolism compared with values before surgery, together with an improvement in glucose and lipid homeostasis and a progressive weight loss [21, 22].
If concomitant growth hormone deficiency exists after pituitary surgery, cardiovascular risk and metabolic and body composition abnormalities worsen even more, all of which may improve after GH replacement therapy [23, 24].
To summarize, imbalance of adipokine production is associated with increased cardiovascular risk and central fat accumulation in CS. Persistent impairment of adipokine secretion may also contribute to the increased long-term cardiovascular risk in patients cured of CS .
Dyslipidemia in CS
According to different series, lipid abnormalities have been observed in 37–71 % of patients with CS, mainly hypercholesterolemia in 16–60 % and hypertriglyceridemia in 1–36 % of patients [25]. Improvements of dyslipidemia after cure/remission occur, but an adverse lipid profile (higher total/HDL cholesterol ratio) can persist in around 30 % of patients, probably due to GC-induced modifications of adipose tissue [2]. However, in a subgroup of subclinical CS patients due to adrenal adenoma, no significant improvements in lipid profile was observed after adrenalectomy [26].
Although the pathogenetic mechanisms of dyslipidemia are multifactorial; insulin resistance and growth hormone deficiency combined with impaired gonadal function can contribute to lipid abnormalities [27]. Given the increased cardiovascular mortality in CS, treatment of dyslipidemia is strongly recommended.
Hypertension and Vascular Damage
Hypertension is one of the most prevalent cardiovascular risk factors in CS, reported in 55–85 % of CS patients, and is associated with the duration of hypercortisolism [4]. Moderately high blood pressure persists despite effective treatment of CS in around 24–56 % of cured CS, mainly when patients are older, had a longer exposure to high levels of GCs, and longer duration of hypertension in the active phase of hypercortisolism . Removal of the source of hypercortisolism led to improvement of hypertension in a significant proportion of patients but not all [28, 29]. Although with a lower prevalence, hypertension, impaired glucose tolerance, and dyslipidemia were still present in a group of cured CS patients; furthermore, a more marked decrease was observed in adrenal adenomas compared to pituitary adenomas [8]. CS patients in remission with persistently high blood pressure have more structural and functional cardiac changes as compared to control hypertensive subjects [30]. Hypertension has also been associated with brain white matter lesions in CS patients in remission [31]. Therefore, it is strongly recommended and often required to prescribe antihypertensive treatment while hypercortisolism exists, as well as in cases in which hypertension persists despite control of hypercortisolism. ACE inhibitors and angiotensin receptor blockers, with their cardioprotective effects, have been recently proposed as a first line treatment [32].
Pathogenesis of hypertension appears to be multifactorial: inhibition of the vasodilating system, activation of the renin–angiotensin–aldosterone system, inhibition of peripheral catecholamine catabolism, increased cardiac output, total peripheral resistance, and renovascular resistance. All these factors together with concomitant insulin resistance and/or sleep apnea are the main contributors to hypertension in CS [32, 33]. Moreover, increased cortisol levels may override the capacity of 11ß-HSD2 (which inactivates cortisol), facilitating cortisol binding to the mineralocorticoid receptor, resulting in an increased effect of aldosterone , that has growth- promoting and profibrotic activities, leading to remodeling and fibrosis of both small vessels and the myocardium [34].
Increased oxidative stress and inflammatory markers (soluble receptor of tumor necrosis factor type 1 (sTNFR1 ), interleukin-6, interleukin-8, glutathione peroxidase, thromboxaneB2, 15-F2t-isoprostane) and decreased antioxidants levels (vitamin E) have been observed in CS compared to controls. These prooxidative processes induced by GCs in combination with metabolic comorbidities lead to a worsening oxidant–antioxidant balance and an increased cardiovascular morbimortality [35]. sTNFR1 has been found to correlate with the Agatston score and to be a predictor of coronary calcifications in a cohort of active and cured CS patients [36]. Also, sTNFR1 has been found to be the strongest predictor of carotid intima media thickness in females with CS [37]. Moreover, endothelin, homocysteine, VEGF, and cell adhesion molecules are increased in active CS patients, while taurine, a suggested protective factor, is decreased. Most of these molecules improved after successful normalization of cortisol levels [38, 39].
An increased carotid intima media thickness and a lower distensibility coefficient were observed in CS after 1 year of remission compared to a BMI-matched control group [2, 40]. The same group observed that atherosclerotic plaques were present in 26.7 % of CD patients compared to <4 % of controls 5 years after remission [2]. Cardiovascular disease was more prevalent in CS patients even after long-term remission (mean time of 11 years); a greater prevalence of coronary calcifications (31 % vs. 21 %) and noncalcified atheroma plaques (20 % vs. 7.8 %), quantified by cardiac multidetector computed tomography (MDCT) angiogram scan, were observed in cured CS compared to age- and gender-matched controls, even after excluding patients with hypopituitarism or dyslipidemia [41]. Also by MDCT , increased coronary calcifications and noncalcified coronary plaque volumes were present in patients with active or previous hypercortisolism, in a small series of mostly ectopic CS [42]. In the same line, atherosclerotic plaques were more prevalent in CS compared to populations matched for similar cardiovascular risk factors, even long-term after remission and they correlated with insulin resistance and central adiposity [43].
Cardiac Morphology: Cardiomyopathy
Several groups have reported functional and structural cardiac lesions such as left ventricular hypertrophy, diastolic dysfunction, and decreased systolic performance in patients with active CS. With remission of hypercortisolemia , cardiac alterations significantly improve, but may not normalize. Myocardial fibrosis has been observed in active CS compared to healthy controls and controls with high blood pressure. Fibrosis appears to be one of the greatest determinants for the degree of regression of cardiomyopathy seen in CS. Nevertheless, successful treatment of CS normalized the extent of myocardial fibrosis, suggesting that hypercortisolism may have a direct effect on myocardial fibrosis independent of left ventricular hypertrophy and high blood pressure [44]. Eighteen months after successful treatment of CS, improvement in left ventricular systolic and diastolic function in parallel to a reduction in myocardial fibrosis was found [45]. In the same line, echocardiographic abnormalities in left ventricular mass parameters were seen in around 70 % of active CS. These abnormalities substantially improved during a mean follow-up of 4 years after the remission of hypercortisolism, although they continued to be more marked as compared to controls [46]. Using cardiac MRI, subclinical systolic biventricular dysfunction together with increased left ventricular mass was found in CS patients compared to controls [47]. After effective treatment of hypercortisolism, an improvement of the systolic performance of both ventricles and reduced left ventricular mass were observed together with a regression of the concentric left ventricular remodeling pattern. This reduction in left ventricular mass was independently associated with changes in glucose metabolism and BMI. Moreover, on the basis of the absence of late gadolinium myocardial enhancement, dense replacement myocardial fibrosis was ruled out in uncomplicated CS [47].
On the other hand, prolonged QTcd (QTc dispersion) in association with ECG evidence of left ventricular hypertrophy seems to be specific features of CD patients and to correlate with hypercortisolemia independently of other cardiovascular risk factors, suggesting a cardiotoxic effect of cortisol excess per se [48]. Also, reduced heart rate variability, an abnormality in cardiovascular autonomic regulation, has been observed in patients with CS; hypercortisolism and disease duration were found to be the main causative factors [49].
Thus, both excess cortisol and high blood pressure contribute to alter cardiac mass and increase the prevalence of damage in target organs. The importance of controlling high blood pressure and other cardiovascular risk factors before surgery to improve long-term prognosis should be emphasized.
In summary, although there is a reduction of fat mass and central obesity after normalization of cortisol, adverse metabolic profile, overweight, and increased cardiovascular risk still persist after remission .
Coagulopathy, Thrombophilia
Cortisol excess induces a procoagulative phenotype (activation of coagulation cascades and impaired fibrinolysis), so that patients with CS have a greater predisposition to thromboembolic events, especially in the perioperative period. This hypercoagulable state in CS is explained by higher levels of procoagulant factors, mainly factors VIII, IX, and von Willebrand factor, as well as an impaired fibrinolytic capacity, due to increase synthesis of the plasminogen activator inhibitor type 1 (the main inhibitor of the fibrinolytic system) [15]. Consequently, there is a shortening of activated partial thromboplastin time and increased thrombin generation [50, 51]. Moreover, both a rise in platelets and endothelial dysfunction observed in patients with CS predispose to increased cardiovascular risk and play a role in the pathogenesis of the prothrombotic state in patients with CS [52, 53]. The incidence of venous thromboembolism (VTE) in CS is higher than in the general population (2.5–3.1 vs. 1.0–2.0 per 1000 persons/year, respectively) [51, 54]. Patients who undergo transsphenoidal surgery for CD have greater risk of thromboembolism than those for a nonfunctional pituitary adenoma, suggesting a role of cortisol (or ACTH) inducing changes in hemostatic factors [54]. Hemostatic and fibrinolytic parameters did not normalize 80 days after biochemical remission with medical therapy [55]. In the same line, in a systematic review the authors observed that even after remission of hypercortisolism , v Willebrand Factor, VII, and IX factors remained high [51]. An improvement in hemostatic parameters after one year of successful surgery has been described, but complete normalization of hemostasis does not occur [56].
In a recent study, an increase risk for VTE (Hazard Ratio, HR 2.6) in patients with CS was found to be already present 3 years before diagnosis, being highest the first year after diagnosis (HR 20.6) and still remained elevated from 1 to 30 years after diagnosis, although most of the cases occurred during persistent hypercortisolism [28].
Although it is still a matter of debate whether systematic antithrombotic prophylaxis in CS should be used, it seems that thromboprophylaxis could be recommended in patients with CS undergoing surgery. However, there is no consensus on the dose or duration of use of prophylactic anticoagulant therapy. Prospective placebo-controlled trials to evaluate the effects of thromboprophylaxis in patients with CD are still lacking.
Additional Hormonal Dysfunction
Remission rates after pituitary surgery can be achieved for 65–100 % of patients. These percentages are lower in patients with a non-visible adenoma, microadenoma with unfavorable localization or macroadenomas and recurrence rates can reach 5–36 % [4]. Secondary hypothyroidism and hypogonadism are common in patients with CS, due to the functional suppression of thyrotropin and gonadotropin secretion by GC excess. After normalization of cortisol secretion, these endocrine abnormalities usually recover, as well as normal menstrual cycles and sexual activity. However, due to structural damage of the residual pituitary gland after surgical removal of the tumor or prolonged inhibition of the hypothalamic–pituitary–adrenal axis, permanent hormone deficiency may occur (hypopituitarism or adrenal insufficiency) [57].
After surgery the most common pituitary insufficiency observed is GH deficiency (which is not always evaluated), followed by thyrotropin and gonadotropin deficiencies [58]. Some patients require life-long replacement with exogenous GC.
GH/IGF1 Axis Impairment : GH Deficiency
GCs are important regulators for GH secretion and action. Prolonged GC excess is a well-known negative regulator of GH secretion. Short stature and delayed linear growth are typical features of pediatric CS, and slowed growth is common in children undergoing long-term high-dose GC therapy. Spontaneous catch-up growth is unlikely even after successful treatment in pediatric CS [59].
There is also evidence supporting the negative impact of hypercortisolism on GH secretion in adult patients. In a group of 34 patients with CD evaluated after long-term remission (median 3.3 years), 65 % presented abnormal GH secretion [60]. The GH/IGF-1 axis recovered at 6 months after successful treatment in half of these patients and was more commonly observed in those patients in whom the HPA axis recovered as well [58].
Interestingly, patients with subclinical hypercortisolism due to adrenal adenomas had a reduced GH secretion reserve compared to patients with nonfunctioning adrenal adenomas after adjusting for age and BMI. In these patients, GH secretion improved after normalization of hypercortisolism [61].
A 3-year follow-up study of GH-treated CD and nonfunctioning pituitary adenomas (NFPA) patients found that in spite of similar prevalence of metabolic syndrome at baseline, metabolic syndrome and cardio- and cerebrovascular disease were significantly higher in treated CD than NFPA patients, suggesting that GHD CD subjects were more predisposed to adverse metabolic features and increased cardiovascular risk [23]. Comparing the effect of GH treatment on lean body mass in cured CD and NFPA patients, NFPA patients showed greater improvement of lean body mass than cured CD after GH treatment, indicating that CD patients could be resistant to the anabolic effect of GH on protein, even years after remission [62].
Assessment of GH secretion is therefore recommended for patients cured from CD, even if not submitted to radiotherapy. Studies on the clinical impact of GH deficiency and the use of GH replacement therapy seem warranted in patients cured from CD .
Bone: Osteoporosis
The prevalence of bone disease, mainly osteoporosis, is high and often underestimated in patients with CS, since not all patients undergo DXA scans, and asymptomatic vertebral and rib fractures can remain undiagnosed. Approximately 30–50 % of CS patients present with fractures, particularly vertebral fractures [3]. Additionally, osteoarthritis and osteonecrosis have been reported mainly in patients with iatrogenic CS, but rarely in patients with endogenous hypercortisolism .
GCs have direct and indirect effects on bone, including decrease osteoblastic and increased osteoclastic activity, reduced intestinal calcium absorption, and increased urinary calcium excretion which induces in both cases a modest increase in parathyroid hormone levels [63]. Deleterious effects on bone, especially on cortical bone microstructure, have been observed using a high-resolution peripheral quantitative computed tomography in patients with active CS [64]. Furthermore, secondary hypogonadism and/or decreases in GH or IGF1 levels induced by excessive amounts of cortisol contribute to the loss of bone mineral density (BMD). The pathophysiology of bone disease in CS is detailed in (Fig. 3) [65].
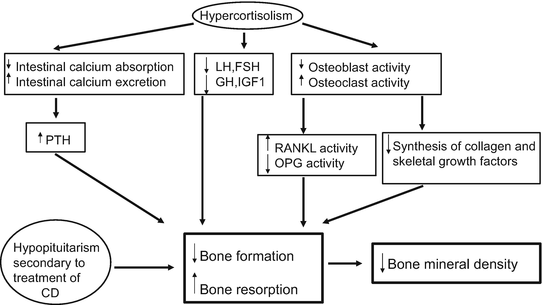

Fig. 3
Pathogenesis of bone disease in CS. (CD: Cushing’s Disease, LH: luteinizing hormone, FSH: follicle-stimulating hormone, PTH: parathyroid hormone, GH: growth hormone, IGF: Insulin-like Growth Factor I, RANKL: receptor activator of NF-Kappa B-Rank-ligand, OPG: Osteoprotegerin)
Studies evaluating bone status after biochemical control of hypercortisolism , however, are often conflicting. While some observed a reversal of GC-induced osteoporosis, others showed an incomplete recovery of BMD and quality of bone after remission. Reversal of GC-induced osteoporosis after long-term remission of CS (mean 72 months) has been described in parallel with increased osteocalcin levels [66]. In the same line, after remission of hypercortisolism, bone mass changes were reversible, probably due to the fact that prior exposure time to endogenous hypercortisolism was shorter than in other studies [67]. The mechanisms causing BMD recovery are speculative. They could be attributed to an increase in osteocalcin levels and to the preservation of trabecular architecture despite the thinning induced by GCs, so osteoblasts may continue synthesizing new bone [68].
A partial recovery of BMD and bone quality after treatment for CS has been reported in most studies (in adolescents and adult patients), although the series are small and median follow-up is relatively short (less than 2 years) [69]. In the series with longer follow-up after remission of hypercortisolism (mean 11 years), decreased BMD values were seen in estrogen-sufficient women as compared to age-, sex- and BMI-matched controls, but not in women with estrogen deficiency, suggesting that the protective effect of estrogens on bone mass is lost with hypercortisolism . Prior exposure time to excess endogenous cortisol and the duration of postoperative GC replacement therapy were predictors of low BMD in these patients [70].
In a group of 50 cured CS, with a median remission time of 13 years, BMD was not significantly different at any site between patients and age- and gender-matched controls. The authors observed that the NR3C1 Bcl1 polymorphism of the GC receptor was associated with reduced total and femoral neck BMD, and patients with ongoing GC replacement presented worse skeletal health (reduced total and lumbar spine BMD) [10].
In summary, BMD recovery appears to be only partial in most patients with “cured” CS .
Myopathy
Around 60–80 % of CS patients present with proximal muscle atrophy and weakness, more frequently in males [3]. GC-induced changes in muscle are evident after a few days of GC exposure or administration, with a more prominent effect on proximal muscles [71]. In aging subjects without CS, muscle mass loss were not associated to circulating or urinary cortisol, but muscle strength correlated with quadriceps expression of 11ß-HSD1, supporting the importance of tissue-specific cortisol metabolism and conversion, rather than overall circulating levels in determining negative effects of GCs [72]. Muscle damage can persist both short- and long-term after cure; it has been related to protein synthesis inhibition and increased rate of protein degradation of myofibrillar and extracellular matrix proteins. Indeed, reduced arm muscle area showed no relevant improvement 6 months after successful treatment, despite a reduction of body fat mass [11]. Reduced lean mass due to muscle loss of limb muscle was observed in CS compared to obese controls with same total body fat mass [73]. In a long-term follow-up, patients with CS had reduced limb skeletal muscle mass, but similar lean body mass compared to age- and gender-matched controls [10]. MRI body composition assessment of CD patients 20 months after remission showed that total and limb skeletal muscle is actually reduced compared to active disease, probably due to the GC replacement therapy after cure [9]. Moreover, postmenopausal women in remission presented with similar muscle mass as active disease patients, suggesting a role of estrogen deprivation in muscle mass as well [9]. Creatinin kinase, plasma myoglobin, and muscle fiber conduction velocity were reduced in the active phase of the disease compared to healthy age-, sex-, and BMI-matched controls and correlated with disease duration [74]. It has been suggested that aerobic and resistance exercises could probably be effective in attenuating GC-induced muscle atrophy [75].
Nephrolithiasis
Nephrolithiasis has been reported in 50 % of active CD and 30 % of cured CD patients compared to 6.5 % in age- and gender-matched controls [4, 76]. The pathogenesis of nephrolithiasis in CS is not fully elucidated. There is probably a synergistic effect of different metabolic changes (hypercalciuria, hypocitraturia, hyperuricosuria, and hyperoxalaturia) together with hemodynamic changes caused by hypercortisolism. In fact, obesity, hypertension, and diabetes, common features of CS, have been seen more frequently in patients with kidney stones. It seems that normalization of cortisol levels can restore the amino acid profile in urine. In a large series investigating the role of different lithogenic factors in CS, high blood pressure and excessive excretion of uric acid were found to be independent risk factors for the recurrence of nephrolithiasis [76, 77].
Cognitive Function and Behavior
Chronic hypercortisolism has been related to changes in memory, behavior, verbal learning, neuronal activity, and other processes of the central nervous system. Psychiatric disturbances have been reported in 54–81 % in different series, major depression and irritability being the most common psychiatric disorders. Emotional lability, mania, paranoia, acute psychosis, anxiety, and panic attacks may also occur in CS [78, 79]. Few reports assess psychopathology after effective surgery; although most of these symptoms and changes improve one year after remission, many persist and do not appear to be fully reversible in the long-term follow-up. An increased prevalence of psychopathology and maladaptive personality traits compared to patients with nonfunctioning pituitary adenomas (NFPAs) and matched controls have been found, indicating that cortisol excess has irreversible effects on the central nervous system, rather than any effect of the pituitary tumor itself [80]. Recently, a retrospective study in a group of patients with CD who underwent bilateral adrenalectomy, with a median follow-up of 11 years, observed improvements in almost all Cushing-specific comorbidities, except for psychiatric morbidities (which included self-reported anxiety, depression, panic attacks, and psychosis ) [81].
On the other hand, the hippocampus, amygdala, and cerebral cortex are important structures involved in cognitive and emotional functions. These structures are rich in GC receptors and, therefore, particularly vulnerable to hypercortisolism. Moreover, 11ß-HSD2 (which inactivates cortisol to cortisone) is not expressed in the hippocampus or limbic structures, which allows the sustained activation of mineralocorticoid receptors by GCs. Since common genetic polymorphism variants in the GC receptor and the 11ß-HSD1 have been recently associated with long-term cognitive impairments in CS in remission (for a median time of 13 years), these results indicate that GC sensitivity and prereceptor regulation of GC action may play a role in the etiology of cognitive dysfunction in these patients [82].
After successful biochemical treatment of CD, psychiatric symptoms may decrease, but patients still show cognitive impairment, decreased quality of life, and a higher prevalence of affective disorders and apathy compared to healthy controls [83–86]. Long-lasting impairments have been reported in several domains of cognitive (attention, visuospatial orienting, alerting, working, verbal and visual memory, verbal fluency, reading speed) and executive functions [83–85]. Higher prevalence of “maladaptive” personality traits in CD, even after long-term cure, has been described [80]. Impaired decision-making together with decreased cortical thickness in selective frontal areas irrespective of the activity of disease has also been observed in CS patients compared to healthy controls, suggesting that chronic hypercortisolemia promotes brain changes which are not reversible after endocrine remission [86]. In the same line, mental fatigue, characterized by mental exhaustion and long recovery time following mentally strenuous tasks, is more common in patients with CS in remission compared to healthy education-, age-, gender-matched controls, according to a very recent study [87].
Decreased hippocampal volume (HV) assessed by 3-T cerebral MRI was seen in CS patients with severe memory impairments compared to controls [83]. Both brain atrophy and reduction in total and cortical grey matter volumes have also been observed in CS compared to controls, but subcortical gray matter reduction has only been seen in those patients with severe memory impairments in parallel to the findings of reduced HV. The negative effects of GC excess on memory and HV seem to be not totally reversible after biochemical cured, since no differences, either in HV or in memory performance between active and cured CS, were found [83]. Brain volumes and cognitive functions have been associated with cardiovascular risk in CS patients in remission [31]. Furthermore, using a proton magnetic resonance spectroscopy, lower N-acetyl-aspartate in the hippocampus (suggesting neuronal dysfunction/loss) and higher levels of glutamate (suggesting glial proliferation as a repair mechanism after neuronal dysfunction) have been observed in cured CS patients compared to matched controls [88]. The authors suggest that these persistently abnormal metabolites could be early markers of GC neurotoxicity, preceding HV reduction [88]. In major depressive disorder patients , similar patterns of reduced HV and reversibility of hippocampal atrophy after treatment have been observed [89]. Moreover, widespread reductions of white matter integrity (reflecting a structural abnormality of white brain matter, like demyelination or loss of axonal integrity) in CD patients with long-term remission (mean 11.9 years) compared with matched controls have also been observed, together with abnormalities in the integrity of the uncinate fasciculus being related to the severity of depressive symptoms [90]. Similarly, structural abnormalities of the brain white matter have been identified with diffusion tensor imaging (DTI) in the brains of CS patients, again suggesting a widespread loss of axonal integrity and demyelination compared to controls [91]. Once present, these alterations seem to be independent of concomitant hypercortisolism, persisting after remission, since a more of these white matter lesions have also been found in CS in remission compared to healthy controls [31, 91]. Moreover, reduced anterior cingulate cortex grey matter volumes and greater volume of the left posterior lobe of the cerebellum in patients with long-term remission of CD (mean 11.2 years) have been observed compared to matched controls [92]. However, another study observed a smaller bilateral cerebellar cortex volume in active CS compared to matched controls [93]. Recently, aberrant resting-state functional connectivity of the brain with the limbic network (responsible for emotional processing and regulation, as well as encoding of memories) and executive control network has been observed in CD patients with long-term remission, suggesting that hypercortisolism may lead to persistent changes in brain functional connectivity (involving episodic memories, semantic knowledge, prospective memory, attention demands, working memory, and cognitive control) [94]. In the same line, altered neural processing of emotional faces after long-term remission of hypercortisolism has been recently reported in CD compared to matched healthy controls [95].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree