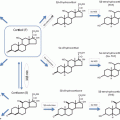
(a) WHO classification
Typical
Atypical
Carcinoma
Histologic features
Ki-67 < 3 %
Ki-67 ≥ 3 %, increased mitoses, extensive p53 staining
Ki-67 ≥ 3 %, increased mitoses, extensive p53 staining
Size
Micro/macroadenoma
Micro/macroadenoma
Macroadenoma
Invasiveness
+/−
+
+
Metastases or craniospinal dissemination
No
No
Yes
(b) Clinicopathologic grading system proposed by the French Collaborative Study [6]
Grade 1
Grade 2
Grade 3
Histologic featuresa
(a) – proliferation
(b) + Proliferation
(a) − proliferation
(b) + Proliferation
+ Proliferation
Size
Micro/macroadenoma
Micro/macroadenoma /giant adenoma
Macroadenoma/giant adenoma
Invasivenessb
−
+
+
Metastases or craniospinal dissemination
No
No
Yes
Aggressive Corticotroph Tumor Subtypes
Three subtypes of corticotroph pituitary tumors that are known to have more aggressive behaviors have been defined (Table 2). First, the Crooke’s cell adenoma (CCA ) represents an entity with the distinctive histologic characteristic of extensive keratin deposition (Crooke’s hyalinization) within greater than 50 % of the corticotroph tumor cells [12, 13]. The CCA is not to be confused with “Crooke’s hyaline change ” which was named after pathologist Dr. Arthur Crooke, who first described the phenomenon of keratin deposition in the normal corticotrophs, an appearance found in the setting of excess glucocorticoid either from diseases such as Cushing’s syndrome or following exogenous glucocorticoid administration [9–11]. CCAs are rare, with only 80 cases reported [12]. This variant of corticotroph tumor presents with ACTH-dependent hypercortisolism similar to other Cushing’s disease cases but is innately aggressive; most present as macroadenomas and exhibit marked cavernous or sphenoid sinus invasion at presentation. Compared to non-Crooke’s cell adenomas, CCAs have a higher recurrence rate that approaches 70 % and more frequently progress to pituitary carcinoma [12]. Among the 36 cases that George et al. reported in 2003, 3 patients (5 %) died of the disease (one from multiple local recurrences and two from pituitary carcinoma) versus 0.01 % death rate for all pituitary tumors [13].
Table 2
Overview of salient features of corticotroph tumor subtypes
Aggressive subtypes | Typical corticotroph tumors | |||
|---|---|---|---|---|
Silent corticotroph adenoma | Crooke’s cell adenoma | Nelson’s syndrome | ||
Histological features | ||||
ACTH immunopositive | ACTH immunopositive | ACTH immunopositive | ACTH immunopositive | |
>50 % tumor cell positive for keratin deposition | ||||
Clinical feature of Cushing’s | ||||
Variably present | Present | Present | Present | |
History of prior BLA | ||||
Biochemical hypercortisolism | ||||
Often absent | Present | Present | Present | |
Spectrum of increased plasma ACTH | ||||
Radiological features | ||||
Size | Often macroadenoma | Micro- or macroadenoma | Macroadenoma | Often microadenoma |
invasiveness | Often present | Often present | Often present | Absent-variable |
recurrence/recurrence rate | Low to medium | High | Recurrence with rapid growth | Low |
Clinical course | ||||
Progress to carcinoma | Limited data but increased | Often | Limited data | Unlikely |
The second type of corticotroph tumors that may exhibit aggressive behavior are the so-called “silent” corticotroph adenomas (SCA). SCAs typically exhibit variable immunoreactivity for ACTH and other pro-opiomelanocortin (POMC)-derived peptides . They may secrete ACTH, which may be elevated in the circulation but often the patient does not exhibit clinical signs or biochemical evidence of hypercortisolism. Rarely, SCAs may transform, in the course of the patient’s disease, into functional adenomas with patients developing clinical hypercortisolism [14]. SCA as a distinct clinicopathologic entity was first proposed in 1978 when the classification of pituitary tumors was based on the tinctorial properties of the tumor cell cytoplasm , i.e., chromophobic, acidophilic, and basophilic. The case that was reported by Kovacs et al. at that time was a densely granulated basophilic cell adenoma which was immunoactive to ACTH antibodies but the patient was eucortisolemic before and after tumor resection [15]. Later, two morphologic variants of SCAs were defined: Type I are densely granulated basophilic tumors similar to functional corticotroph tumors whereas type II are chromophobic with varying ultrastructural patterns [16]. The morphological difference suggests that there might be variations in clinical phenotype resulting from these SCAs, but due to the small number of cases available, studies have mostly examined the collective features of the SCAs . Initially the mechanism proposed for the “silent” biochemical and clinical features of these tumors invoked impaired ACTH synthesis with enhanced lysosomal degradation of POMC peptides [15]. More recent studies have demonstrated the incomplete processing of POMC, the precursor peptide of ACTH due to reduced expression of the prohormone convertase (PC1) enzymes PC1-3 [16–18]. Additionally the corticotroph-specific transcription factor TPIT was found to be lower in several SCAs, suggesting altered corticotroph differentiation in at least some of these tumors [17]. Due to the “silent” clinical course of these tumors, many are found as incidentalomas after brain imaging for other reasons or when patients present with symptoms of mass effect [19]. In a large surgical series, most SCAs were macroadenomas with suprasellar extension present in 87–100 % of cases, and compared to nonfunctional adenomas and functional ACTH-secreting tumors, SCAs exhibited a more aggressive clinical course with frequent recurrence [20–23]. Other retrospective reviews from individual institutions reported similar recurrence rates in SCAs as nonfunctioning adenomas [24, 25], but the pace of regrowth tended to be more aggressive [25]. Some patients with SCAs have been noted over time to manifest clinical signs and biochemical evidence of hypercortisolism. Whether this represents a true transformation of the tumor or more likely in the opinion of the authors the tumor as it enlarges attains a threshold of partially active ACTH secretion that can bind the ACTH receptor sufficiently to induce glucocorticoid excess is unclear .
The third setting where corticotroph tumors may behave aggressively is in the setting of Nelson’s syndrome . In 1958, Dr. Nelson reported the development of an ACTH-secreting pituitary tumor following bilateral adrenalectomy (BLA ) [26] and an early case series in 1979 found that 4 of 12 patients (33 %) treated with bilateral adrenalectomy for Cushing’s disease developed pituitary corticotroph tumor growth (Nelson’s syndrome). Two out of the 4 patients had spontaneous tumor infarction, one patient died from local tumor invasion despite radiation therapy and another patient had corticotroph tumor regrowth after surgical resection [27]. Nelson’s syndrome reminds us of the role of glucocorticoid-mediated negative feedback to control pituitary corticotroph tumor growth whereby removal of cortisol-mediated negative feedback on the pituitary tumor serves as a growth stimulus [28]. A variety of risk factors have been implicated for corticotroph tumor growth after bilateral adrenalectomy including the presence of radiographically visible pituitary tumor remnant, young patient age, duration of Cushing’s disease and lack of pituitary radiation prior to BLA. A recent study of 53 patients with Cushing’s disease found that short duration of Cushing’s before BLA and high plasma ACTH level in the year following BLA were independent predictors for pituitary corticotroph tumor progression, the latter most likely to occur within the first 3 years after BLA [29].
Aggressive Corticotroph Tumors: Role of Histopathological Indicators
As noted in the introduction, the 2004 WHO criteria list Ki-67 ≥ 3 % and extensive p53 immunostaining as indicators of an atypical pituitary tumor or carcinoma. Ki-67 is a well-validated marker expressed during the G1, G2-M, and S-phase of the cell cycle. Commonly detected by the monoclonal MIB antibody it is reported in the form of Ki-67 labeling index (LI) , indicating the number of Ki-67 positive cells in either 4 × 200 high-powered fields or by less standardized approaches (see later). Pituitary tumors exhibit a very broad range of Ki-67 LI with the vast majority of pituitary adenomas exhibiting Ki-67 LI between 1 and 2 % [30, 31]. In an early study based on 77 cases, Thapar et al. demonstrated significant differences in Ki-67 LI among 37 noninvasive tumors, 33 invasive tumors, and 7 pituitary carcinomas. The authors proposed that a threshold LI of 3 % could be used to distinguish invasive from noninvasive adenomas with 97 % specificity and 73 % sensitivity [5]. This threshold of 3 % was ultimately used in the WHO classification to differentiate atypical pituitary adenomas and carcinomas from typical pituitary tumors. However, prospective studies supporting this cutoff are lacking, and the utility of Ki-67 LI to robustly distinguish benign/typical versus invasive/atypical adenomas is not universally accepted [8, 30, 31]. For example, although one study found that Ki-67 LI was significantly higher in ACTH-secreting tumors versus other functional or nonfunctional tumors [32], that finding was not supported by other studies and despite increased growth in the setting of Nelson’s syndrome as previously discussed, no significant association was found between Ki-67 LI and tumor recurrence in patients with Cushing’s disease compared to tumors from patients with Nelson’s syndrome [33]. Furthermore, the mean Ki-67 LI was relatively low at 0.7–0.8 % in 11 primary Crooke’s cell adenomas, although Ki-67 LI was higher at 2.1–6.1 % in the recurrent Crooke’s cell tumors . These mostly small single center studies must be interpreted with caution but would appear to highlight limitations of the Ki-67 LI as a stand-alone predictive marker of corticotroph tumor aggressiveness [13].
P53 is a cellular tumor antigen that plays an important role in genomic stability and cell proliferation. In the Thapar study above p53 immunoreactivity was also reported to correlate with pituitary tumor invasiveness and was expressed in 100 % of pituitary carcinoma cases [5]. However, subsequent studies have not observed a clear-cut association between p53 and invasiveness of pituitary tumors [32, 34, 35]. This in large part may be due to the considerable intra and intertumoral variability of p53 tumor expression and we must conclude that the independent role of p53 in predicting pituitary tumor behavior is quite limited.
The situation for these and other immunohistochemical markers i s further complicated by the method of analysis for Ki-67 LI which is not standardized. Some pathologists “eyeball” the Ki-67 LI on analyzing variable numbers of tumor sections, more standardized quantitation methods (4 × 200 high power fields) are labor intensive and computed quantitation analysis is not universally available and may overestimate by counting infiltrating Ki-67 false positive inflammatory cells.
In summary, while the prognostic values of Ki-67 and p53 staining remain controversial, they are presently the most readily available tools to clinicians and it is prudent to monitor corticotroph and other pituitary tumors with Ki-67 LI outside the norm, i.e., >2–3 % more vigilantly.
Emerging Molecular Markers of Corticotroph Tumor Invasion and Aggression
The exact pathogenesis of pituitary tumors including aggressive pituitary corticotroph is not fully understood but significant advances have been made in the past decade to further our understanding of the transformation of “benign” pituitary tumors to aggressive tumors and pituitary carcinoma. At present, there is no single biomarker that faithfully predicts pituitary tumor behavior [36, 37]. Multiple pathways, including occasional genetic mutations , dysfunctional hormonal and growth factor signaling pathways cooperate to promote pituitary tumor cellular proliferation. Several biomarkers of pituitary tumor aggressiveness have been implicated, though it is important to note the majority are not unique to corticotroph tumors.
For example, invasive pituitary tumor s express higher levels of matrix metalloproteinases (MMPs ), a class of proteinases that play a key role to break down basement membranes and connective tissues to enable tumor cell access to the extracellular environment. They can also coactivate other family members whereby MMP-2 activates MMP-9 [38]. In a small study, 9 of 10 (90 %) invasive pituitary tumors exhibited functional polymorphisms in the promotor region of the MMP-1 gene resulting in increased MMP-1 transcriptional activity [39, 40]. Additionally, expression of MMP-9 which degrades collagens, elastin, and gelatin was found to be higher in invasive pituitary adenomas [41–44]. In turn MMP-9 can serve to activate protein kinase C (PKC) further contributing to corticotroph tumor aggression [44].
The fibroblast growth factors (FGFs ) and their receptors (FGFR) regulate growth, differentiation, migration, and angiogenesis. High levels of FGF mRNA and circulating FGF-2 levels have been reported in aggressive pituitary tumors. Reduction of β-catenin expression resulting in loss of cytoskeletal integrity has been implicated in the process. Also, FGFR4-R388, an FGFR4 allele associated with poor cancer prognosis, was found to be associated with MMP [45].
Vascular endothelial growth factors (VEGFs) and their receptors (VEGFRs) are key signaling proteins essential for tumor angiogenesis. Small case series have reported that pituitary tumors with high VEGF expression have a higher risk of extrasellar growth and recurrence [46]. In support for a role of the VEGF pathway in pituitary tumor aggressiveness, a study of 95 pituitary tumors found that lower expression of an inhibitor of VEGF, called vascular endothelial cell growth inhibitor (VEGI) was associated with suprasellar and sella destruction [47]. A further study reported higher expression of endocan, a proteoglycan involved in neoangiogenesis, in invasive pituitary tumors [48, 49].
Although classic oncogenic mutations such as Ras mutations are uncommon in pituitary tumors [55], a variety of inherited mutations have been implicated in pituitary tumorigenesis. For example, pituitary tumors, including corticotroph tumors, are found in multiple endocrine neoplasia type I (MEN 1) and familial pituitary adenoma (FIPA) . Some studies have reported that pituitary corticotroph adenomas in those inherited conditions may be larger and more often invasive than sporadic tumors [50, 51]. Both the MEN1 and aryl hydrocarbon receptor interacting protein (AIP) genes are located on chromosome 11q13 [52, 53]. Allelic deletion of 11q13 and an additional 3 loci (13q12–14, 10q, and 1p) and dysregulation of chromosome 11p was found to be more common in aggressive pituitary tumors [48, 54]. Studies in small numbers of invasive versus noninvasive prolactinomas identified ADAMTS6, CRMP1, and DCAMKL3 to be associated with invasion and ASK, CCNB1, AURKB1, CENPE, and PTTG with proliferation [56]. Pituitary tumor transformation gene (PTTG ), for example, a member of the securin protein family that regulates sister chromatid separation during mitosis, has been studied extensively and shown to correlate with invasion in several tumor types including corticotroph tumors [57].
Most recently, mutations in ubiquitin-specific protease 8 (USP8) , a gene coding a deubiquitinase that inhibits lysosomal degradation of epidermal growth factor receptor (EGFR) , were identified in 40 % of corticotroph tumors [58]. Additionally overexpression of the heat shock protein 90 (HSP90) that alters glucocorticoid receptor folding thereby inducing glucocorticoid resistance was demonstrated in corticotroph tumors [59]. However, it is as yet unclear if either USP8 or HSP90 correlates with corticotroph tumor aggressiveness. Potentially, future molecular and histological analysis with established factors such as Ki-67 and p53 could be enhanced with integration of some emerging biomarker candidates such as MMP, PTTG, miRNAs, and chromosome deletion in 11p and 11q. However, the practical application of these biomarkers in routine clinical use as opposed to research studies has not yet been examined.
Role of Surgical Debulking/Resection in Aggressive Corticotroph Tumors
Surgical approaches to either obtain complete near-total resection or significant debulking remain first line therapy in the majority of corticotroph tumors. The wider exposure obtained and the enhanced direct visualization that angulated endoscopes provide may facilitate a more extensive surgical resection of tumors that extend beyond the sella into the cavernous sinuses and other parasellar structures. Occasionally a transcranial approach may be needed in tumors that extend significantly into the suprasellar region. With exceptions, aggressive corticotroph tumors tend to be invasive macroadenomas from presentation, and although it may be possible to achieve a visualized total resection with postoperative imaging showing “no residual tumor,” these aggressive corticotroph pituitary tumors tend to recur, typically within 5 years [31]. As noted in prior sections, histopathology assessment may raise the possibility of tumor aggression, alerting the clinician to closely monitor the patient both biochemically (cortisol and ACTH parameters) and by imaging. As in other corticotroph tumors, low (<5 μg/dL) immediate postoperative serum cortisol is a good indicator of immediate remission [60–63]. Thereafter, patients require glucocorticoid replacement for typically 6–12 months. If a patient is able to stop glucocorticoid replacement sooner, this raises concern that they have not ever been fully in remission or have had early recurrence, the latter a potential clinical indicator of an aggressive corticotroph tumor.
Radiation Therapy
Whereas radiation therapy (RT) is not usually effective to induce corticotroph tumor shrinkage it can be helpful to prevent regrowth in subtotally resected corticotroph tumors or to slow growth of an expanding sellar lesion. Radiation can be delivered either as stereotactic radiosurgery (SRS) which involves delivery of high dose radiation typically in a single dose offering good efficacy and enhanced patient convenience, or in small daily dose fractions (fractionated RT) over 5–6 weeks [65]. Fractionated RT is particularly helpful when the tumor approximates radiation sensitive normal tissues that cannot be spared from the RT field. Various forms of radiation therapy exist, including gamma-knife, linear accelerator, cyber-knife, and proton beam therapy that can all be adapted to deliver either SRS or fractionated RT. To date, the greatest experience with SRS has been with gamma knife. Comparing success rates of the various radiation treatments is challenging due to differences in technique, doses administered, duration of follow-up, and definitions of tumor control and biochemical remission [66]. That said, a large retrospective single institution review of proton beam RT showed that actuarial 3-year biochemical remission was achieved in 54 % of 74 patients with persistent Cushing’s disease and in 63 % of 8 patients with Nelson’s syndrome . Time to biochemical remission was 32 months and 26 months, respectively, and tumor control was achieved in 98 % of the patients with Cushing’s disease. The main adverse effect is panhypopituitarism which eventually occurred in 62 % of the 140 patients studied [67]. In another retrospective study involving 96 patients with persistent Cushing’s disease treated with gamma knife RT after surgery, 70 % of patients achieved biochemical remission at a median follow-up of 48 months. Median time to remission was 16.6 months and tumor control was achieved in 98 % of patients [68]. As noted in these studies, an additional challenge of RT is delayed biochemical remission, necessitating use of medical therapy until radiation therapy controls the hypercortisolism.
Medical Management
Aggressive corticotroph pituitary tumors similar to any ACTH-secreting tumors may cause complications of hypercortisolism including hyperglycemia, hypertension, venous thromboembolism, and poor wound healing resulting in significant morbidity and mortality. Therefore effective control of hypercortisolism is of paramount importance at all stages in managing these patients, including across potentially definitive therapies such as radiation treatment. Several medical treatments aimed at lowering cortisol levels are currently available [69–71]. An ideal therapy would simultaneously lower ACTH and cortisol levels and offer tumor control with minimal side effects, but no such agent presently exists.
Medical therapies that act at the site of the tumor include the dopamine receptor-2 agonist cabergoline which is generally well tolerated and given its ease of administration can be considered as a medical option for aggressive corticotroph tumors. In patients with Cushing’s disease, cabergoline normalized 24-h urinary free cortisol in 40 % of 18 patients and resulted in tumor shrinkage in 4/8 patients treated with doses ranging from 1 to 7 mg/week for 12–24 months [73–75]. However, most would consider D2 agonists weak anti-proliferative agents in corticotroph tumors.
Octreotide, a first generation somatostatin (SMS) analog predominantly targeting the somatostatin receptor subtype-2 (SSTR-2) has been reported to lower ACTH levels and stabilize tumor progression in some patients with Nelson’s syndrome [76], but no consistent effect of octreotide is found in patients with Cushing’s disease [77, 78]. Pasireotide (SOM 230) , a somatostatin receptor ligand with higher binding affinity for SSTR-5, normalized 24-h urinary free cortisol in 20 % of patients with Cushing’s disease [79, 80]. Data regarding the action of this agent on corticotroph tumor growth are awaited.
An alternate method to lower serum cortisol is the use of either adrenal steroidogenesis inhibitors such as ketoconazole, metyrapone, and mitotane or the glucocorticoid receptor (GR) antagonist mifepristone.
In one study of 38 patients, 21 of who had not undergone prior pituitary surgery ketoconazole treatment (200–1200 mg/day) normalized 24-h urinary free cortisol in 45 % of patients [81]. A large retrospective multicenter French study similarly reported normalized urinary free cortisol in 49 % of 200 patients [82]. Side effects include nausea, diarrhea (8 %), and skin rash (2 %), and gynecomastia in men (13 %). It is important to point out that ketoconazole like all adrenal- or GR-directed agents will not inhibit tumor growth but nonetheless can be very effective in controlling symptoms of hypercortisolism in combination with other therapies directed at tumor control. Metyrapone is also effective in controlling hypercortisolism. In one study normalization of 24-h urinary free cortisol was reported in 39 of 53 patients (75 %) with Cushing’s disease after 1–6 weeks using a mean dose of 2250 mg [83]. Similar response rates were reported in a more recent UK study of 195 patients [84]. As for ketoconazole , gastrointestinal side effects of metyrapone predominate. Hirsutism and acne (70 %) due to androgen accumulation, as well as hypertension and edema (70 %) due to 11-deoxycorticosterone accumulation, can also be seen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree