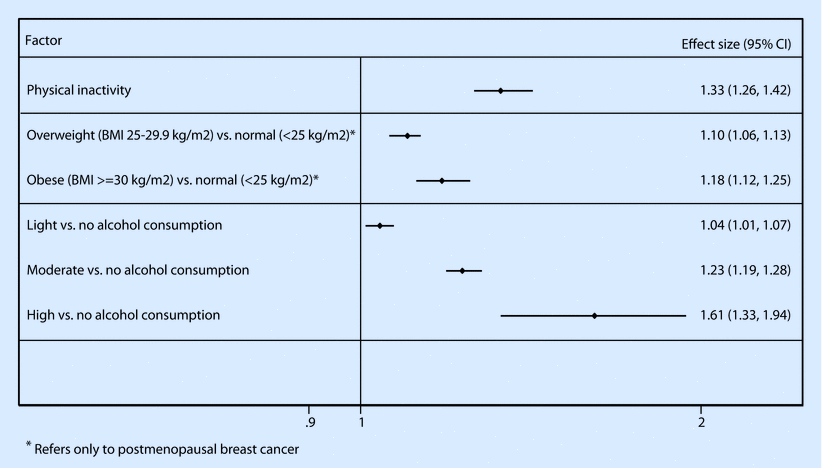
Fig. 4.1
Dose-response meta-analysis for the association between BMI and risk of breast cancer in postmenopausal women (From Xia et al. [60]
Weight Change
A number of studies have assessed the effect of weight change during lifetime on breast cancer risk. The majority of studies pertain to postmenopausal breast cancer and show an increased risk with weight gain after 18 years of age; the effect over adulthood is estimated at 5% per 5 kg of weight gain [56, 64]. Furthermore, weight gain after menopause has been also found to independently increase breast cancer risk (OR: 1.18 for weight gain of at least 10 kg compared to women with no weight change) [64]. It is worth noting, however, that periods of potential weight loss have not been taken into account. The published data for premenopausal breast cancer are limited. In one report no effect was found [65], whereas another study showed a marginally significant decrease in breast cancer risk among women reporting weight loss since the age of 18 years [66].
Central Adiposity
Of interest is also the association of breast cancer with fat distribution, besides overall adiposity. Central adiposity is linked to metabolic-hormonal changes leading to insulin resistance, as well as hyperandrogenemia and excess conversion of peripheral androgens to oestrogens. Waist-to-hip ratio is considered a reliable measure of central adiposity and has been consistently associated with postmenopausal breast cancer [56, 67]. Again findings for premenopausal breast cancer remain inconsistent and inconclusive; as contrasted to BMI, however, two meta-analyses have shown an increased risk of premenopausal cancer in women with central adiposity [67, 68]. The results were heterogeneous, though, whereas the effect seemed to be stronger among women of Asian origin.
4.2.2 Exercise
Physical activity is considered an established primary breast cancer prevention strategy for both premenopausal and postmenopausal women [69]; indeed, sustained regular activity throughout life seems to provide the highest benefit [70]. Noticeably, it has been estimated that elimination of physical inactivity would result in a decrease of breast cancer incidence by 10% [71]. The first study aiming to assess this association, using a detailed lifetime physical activity assessment, showed a 58% decreased risk of breast cancer among premenopausal women with an average of 3.8 h of exercise per week, compared to those who reported no exercise [72]. Similarly, a decreased risk by 45% for breast cancer in postmenopausal women in the highest physical activity category was found [73]. A large recent meta-analysis summarizing evidence from 31 cohort studies confirmed the results and showed an overall 13% decrease in risk among women reporting the highest versus the lowest level of physical exercise. A dose-response pattern was recorded, and the overall estimate was stronger for premenopausal women and women with normal BMI values, compared to overweight, and confined to oestrogen/progesterone receptor-negative tumours [74]. Interestingly, in a systematic review of the literature, occupation-related, household, recreational and walking-associated physical activity all seemed to independently decrease the risk for breast cancer [75].
4.2.3 Underlying Mechanisms for Obesity and Exercise
The adverse effects of obesity and the beneficial actions of physical activity are generally considered to be exerted via common mechanisms. The proposed actions could be generally synopsized as (a) exposure to oestrogens, (b) changes in insulin-related factors and adipokines and (c) anti- or pro-inflammatory actions and other protective mechanisms. These are described below.
Exposure to Oestrogens
Body fat in postmenopausal women is a main source of oestrogen secretion via aromatization of androgens [76]. Therefore, the higher oestrogen levels in obese postmenopausal women seem to be responsible for the increased risk of breast cancer after menopause; similarly, the observed protective effects of physical activity seem to be mainly mediated by the subsequent body fat loss associated with regular exercise in this age group.
Physical activity, in premenopausal women, where the association between adiposity and breast cancer risk is reversed, seems to act via independent mechanisms. Specifically, high levels of physical exercise in reproductive years are associated with reduced levels of circulating oestradiol and progesterone, possibly because of irregular or anovulatory menstrual cycles or a shortened luteal phase. Lastly, in puberty, vigorous activity could delay the onset of menarche, which has been associated with a decreased risk of breast cancer.
Downregulation of Insulin-Related Factors
Physical activity may prevent breast tumour development by lowering levels of the hormones insulin and insulin-like growth factor-I (IGF-I) [77]. Decreased insulin upregulates sex hormone-binding globulin (SHBG), leading to lower levels of bioavailable oestradiol [78]; it also seems to decrease the bioavailability of IGF-I [79]. IGF-I acts as a mitogen in breast epithelial cells, promoting transformation and suppressing apoptosis [80]; consequently, lower circulating levels of IGF-I are associated with a decreased breast cancer risk [81].
Benefits on Obesity-Related Adipokine Levels
Adipose tissue is considered an endocrine organ which produces and secretes adipokines, involved in the mediation of inflammatory diseases and obesity. In a recent study, adiponectin, leptin, visfatin and resistin were all found to be risk factors for breast cancer in postmenopausal females. In particular, leptin, resistin and visfatin levels were positively correlated with TNM staging, tumour size, lymph node metastasis and histological grading in postmenopausal subgroups [82]. As a result, physical exercise and its inhibitory effect upon adipose tissue provide downregulatory signals for decreased adiponectin secretion.
Anti-inflammatory and Other Protective Mechanisms
Chronic inflammation is now considered a key process in carcinogenesis, and physical activity has been known to suppress increased levels of inflammation, whereas obesity is now recognized as a chronic inflammatory disorder [83, 84]. Other proposed protective mechanisms of exercise against breast cancer risk include an exercise-induced decrease in breast density, reduction in oxidative stress, enhancement of immune function, promotion of tumour suppressor genes and intracellular anticarcinogenic pathways [85].
4.3 Effects of Diet
Diet has been associated with the development or progression of major human cancers, including breast, prostate and colorectal tumours. It is a modifiable factor, which has been the subject of intensive research interest as it provides a window for preventive intervention strategies, due also to the immense interest of lay people in associating their dietary habits with cancer risk and breast cancer risk, in particular.
4.3.1 Macronutrients and Food Items
Among macronutrients and food items, dietary fibre has been proposed to reduce breast cancer risk via decreasing reabsorption of oestrogens in the gastrointestinal system [86], yet evidence remains inconclusive [87, 88]. Regarding fat intake, there are experimental studies suggesting that an increased fat intake could induce mammary gland tumours in rodents [89]. On a molecular level, fatty acids could influence the carcinogenic process through mechanisms related to modifications of cell membrane structure, metabolic effects and impact on translational signals and gene expression [87]; findings from humans, however, do not seem to strongly support this hypothesis [87, 88, 90]. Regarding other food groups, high dietary intake of carbohydrates has been suggested to increase breast cancer via induced hyperinsulinemia.
Many studies on the association of specific dietary factors with breast cancer have been published showing conflicting results, notably inverse, positive or null associations [91]. Briefly, consumption of fruit, vegetables and fish, as well as soy-based food and isoflavone intake, seems to be associated with decreased breast cancer risk, possibly on account of their antioxidant effects and the decrease in oestrogen levels related to soy-based food. Meat intake, apart from being associated with colorectal cancer, has been also found in some studies to increase breast cancer risk, but the evidence is limited [87, 88].
4.3.2 Dietary Patterns
A lower breast cancer incidence has been encountered by women who live in Mediterranean countries compared to those in Northern European countries, such as the UK or the USA; in this context, the Mediterranean pattern of diet has been considered as a tentative contributing factor. In the small MeDiet study, study subjects were randomized into a dietary intervention group (n = 58) following a traditional, controlled Mediterranean diet for 6 months and a control (n = 57) group, which continued to follow their regular diet. At baseline, no significant difference was observed in urinary levels of individual oestrogens comparing intervention and control women. After 6 months, however, whereas no major change was noted in the control group, as expected, women in the intervention group showed a significant reduction (over 40%, p < 0.02) of total oestrogen levels [92]. These findings need to be further confirmed in the hope that this strategy may be part of breast cancer prevention strategies. A number of studies have also explored whether the so-called Western diet, traditionally including high red and processed meats, refined grains, potatoes and starches, snacks, sweets, fried foods and soft drinks, impacts on breast cancer risk; the majority, however, have not found statistically significant associations [87].
4.3.3 Micronutrients
Evidence regarding the association of micronutrients with breast cancer risk in both premenopausal and postmenopausal women is not consistent. Despite the reported antioxidant effects of vitamin C, vitamin E and selenium, most cohort studies do not confirm these results. By contrast, the protective effect of vitamin A and retinol intake seems to merit further research [93] taking into account variations in absorption, metabolism and excretion of carotenoids between individuals [94].
Regarding other micronutrients, higher dietary folate intake has been associated with decreased breast cancer risk, especially among women reporting higher alcohol consumption indicating an interactive effect [95]. Similarly, a higher dietary intake of methionine, but not vitamin B6 and B12, has been proposed to have an inverse association, especially for postmenopausal women [96]. These molecules are considered essential for DNA synthesis, repair and methylation, and their deficiency could lead to DNA instability and proneness to carcinogenic mutations [97]. Lastly, there are some suggestions of an inverse association between vitamin D, as well as calcium intake and breast cancer risk [98, 99], which were not confirmed, however, in the results of a randomized trial assessing the link between use of combined calcium and vitamin D supplementation with breast cancer risk [100].
4.3.4 Alcohol Consumption
Alcohol consumption is considered to be causally related to breast cancer risk, with a 7–10% increase in risk for each 10 g (~1 drink) of alcohol daily consumption by premenopausal or postmenopausal women [101]. A dose-response pattern is noted with heavy drinking increasing the risk by approximately 60% and enhanced associations for hormone receptor-positive tumours [102]. Proposed mechanisms include the effect of alcohol on circulating oestrogen levels and the carcinogenic role of ethanol metabolites or the effect of alcohol on epithelial-mesenchymal transition, epithelium-stroma interaction and epigenetic regulation of gene expression in the breast [101]. Alcohol consumption may cause a rise in oestrogen levels by promoting aromatase activity that converts androgens to oestrogens, inhibiting oestrogen degradation, decreasing melatonin secretion that inhibits oestrogen production and increasing hepatic redox state that results in a decrease in steroid metabolism. Additionally, acetaldehyde and free radicals, alcohol metabolites, may cause DNA damage promoting carcinogenesis. Lastly, the decrease in folate levels due to antagonism by alcohol may contribute to breast cancer development [101].
4.3.5 Non-alcoholic Beverages
Caffeine was implicated in breast tumorigenesis, on account of an observation that coffee consumption reduction was associated with regression of fibrocystic breast disease [103]. The results of subsequent large cohort studies are synopsized in a recent meta-analysis [104] showing no association. Of note, however, are suggestions for a small inverse association specific for green tea consumption and its association with breast cancer incidence and recurrence, possibly on account of its antioxidant actions [105], which does not apply for black tea consumption [106].
4.4 Conclusions
Breast cancer remains the leading type of malignancy in women worldwide with a profoundly higher incidence in the developed compared to developing countries. Increasing trends have been evident in Western communities for several decades. Lifestyle changes leading to obesity and physical inactivity, higher alcohol consumption and variable use of exogenous oestrogens are believed to have contributed to the observed increase of the disease. Higher oestrogen exposure, indicated by proxies, such as earlier age at menarche, later menopause and adverse childbearing patterns, has been witnessed in parallel [107]. There is no strong evidence that other macro- or micronutrients are clearly included in the aetiology of the disease.
Intense research over the last few decades has broadened our understanding of the aetiology of the disease and revealed modifiable factors offering applicable preventive targets (◘ Fig. 4.2). According to the World Cancer Research Fund, 40% of postmenopausal breast cancers would have been prevented, where it is possible to reduce alcohol consumption, physical inactivity and obesity [41]. In order to formulate public health recommendations and tangible prevention guidelines, future research should focus on specific components of modifiable risk factors, such as type and timing of physical activity, and their geographical, ethnical and cultural customization. Indispensable is further exploration of the model of oestrogen- or other hormone-induced carcinogenesis, the findings of which should be clinically integrated into breast cancer prevention and control programmes.
Key Points
Oestrogens are involved in breast carcinogenesis by exerting proliferative effects on breast epithelial tissue.
High levels of serum oestrogens have been linked with increased risk of breast cancer, especially among postmenopausal women.
Proxies of high lifetime exposure to endogenous oestrogens, like early menarche and late menopause, are associated with increased risk of breast cancer, whereas premenopausal oophorectomy seems to decrease the subsequent risk. Childbearing and young age at first pregnancy decrease the lifetime risk for breast cancer.
Well-documented findings support the finding that the use of combined oestrogen-progesterone hormone replacement therapy (HRT) increases the risk for breast cancer in postmenopausal women.
Oral contraceptives, ovarian stimulation for infertility treatment and hormone therapy for transgender individuals do not seem to increase breast cancer risk.
A bimodal pattern has been observed regarding the association of obesity with breast cancer, with an increased risk for postmenopausal and a decreased risk for premenopausal women.
Physical exercise decreases the risk of breast cancer in a dose-response pattern, thus comprising a target for preventive strategies.
Body fat comprises the main source of oestrogens in postmenopausal women via aromatization of androgens, and this is thought to be the main mechanism underlying the aggravating effect of obesity, as well as the protective action of exercise. Other mechanisms include the IGF-I system, adipokines and inflammation.
Alcohol consumption is a well-established risk factor for breast cancer.
There is no consistent evidence regarding association with other dietary patterns.
References
1.
Feigelson HS, Henderson BE. Estrogens and breast cancer. Carcinogenesis. 1996;17(11):2279–84.PubMed
2.
Key TJ, Appleby PN, Reeves GK, Travis RC, Brinton LA, Helzlsouer KJ, et al. Steroid hormone measurements from different types of assays in relation to body mass index and breast cancer risk in postmenopausal women: reanalysis of eighteen prospective studies. Steroids. 2015;99(Pt A):49–55.PubMed
3.
Key T, Appleby P, Barnes I, Reeves G, Endogenous H. Breast cancer collaborative G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–16.PubMed
4.
Sieri S, Krogh V, Bolelli G, Abagnato CA, Grioni S, Pala V, et al. Sex hormone levels, breast cancer risk, and cancer receptor status in postmenopausal women: the ORDET cohort. Cancer Epidemiol Biomark Prev. 2009;18(1):169–76.
5.
Endogenous H, Breast Cancer Collaborative G, Key TJ, Appleby PN, Reeves GK, Travis RC, et al. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 2013;14(10):1009–19.
6.
Collaborative Group on Hormonal Factors in Breast C. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141–51.
7.
Haslam SZ. The ontogeny of mouse mammary gland responsiveness to ovarian steroid hormones. Endocrinology. 1989;125(5):2766–72.PubMed
8.
Madigan MP, Troisi R, Potischman N, Dorgan JF, Brinton LA, Hoover RN. Serum hormone levels in relation to reproductive and lifestyle factors in postmenopausal women (United States). Cancer Causes Control. 1998;9(2):199–207.PubMed
9.
Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas. 1995;21(2):103–13.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree