Chapter 14 Efavirenz
Efavirenz (EFV, Sustiva) is a human immunodeficiency virus type 1 (HIV-1) specific, non-nucleoside reverse transcriptase inhibitor (NNRTI). EFV is a noncompetitive inhibitor of HIV-1 reverse transcriptase (RT) and has no inhibitory effect on HIV-2 RT. EFV binds directly to RT and inhibits viral RNA- and DNA-dependent DNA polymerase activities by disrupting the catalytic site. Although the drug–RT–template complex may continue to bind deoxynucleoside triphosphate and to catalyze its incorporation into the newly forming viral DNA, it does so at a slower rate.1–3
It was approved by the US Food and Drug Administration (FDA) in 1998 for the treatment of HIV in combination with other antiretroviral medications in adults and children over 3 years of age. It is available as 200 mg capsules, 600 mg tablets, and as a liquid formulation.4,5
EFV plus lamivudine in combination with zidovudine, tenofovir DF, or stavudine is the preferred NNRTI-based regimen for HIV treatment-naive patients. It can also be used as part of a combination therapy for salvage regimens for treatment-experienced patients.6
EFV may be used with other antiretroviral agents as part of an expanded postexposure prophylaxis regimen for healthcare workers and other individuals exposed occupationally to tissues, blood, or other body fluids associated with a high risk for HIV transmission. EFV should be considered when resistance to protease inhibitors (PIs) in the source person’s virus is known or suspected.7
EFV is an FDA pregnancy category D drug. Women should not become pregnant or breastfeed while taking EFV. Serious birth defects, mostly involving neural tube development, have been seen in children of women treated with EFV during the first trimester of pregnancy. Women must use a reliable form of barrier contraception, such as a condom, even if they also use other methods of birth control (see section on Pregnancy).4,6
STRUCTURE
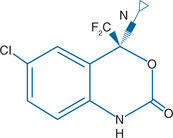
EFV is chemically described as (S)-6-chloro-4-(cyclopropylethynyl)-1, 4-dihydro-4-(trifluoromethyl)–2H-3, 1-benzoxazin-2-one.3 Molecular mass of the compound is 315.68. EFV has the empirical formula C14H9CIF3NO2 and the structural formula is shown in Figure 14-1.
IN VITRO ACTIVITY AND MECHANISM OF ACTION
EFV activity is mediated predominantly by noncompetitive inhibition of HIV-1 RT. HIV-2 RT and human cellular DNA polymerases alpha, beta, gamma, and delta are not inhibited by EFV. EFV binds directly to RT and inhibits viral RNA- and DNA-dependent DNA polymerase activities by disrupting the catalytic site.1 The 90–95% inhibitory concentration (IC90–95) of EFV for wild-type laboratory strains and clinical isolates ranged from 1.7 to 25 nM.
PLASMA PHARMACOLOGY
Peak plasma concentrations (Cmax) of 1.9–9.1 μM were achieved in healthy individuals within 5 h after a single oral dose of 100–1600 mg of EFV. Dose-related increases in Cmax and area under the time–concentration curve (AUC) were seen for doses up to 1600 mg, however, as the dose increased, the absorption decreased. The increases were less than proportional suggesting diminished absorption at higher doses.2,3
In HIV-infected individuals, mean minimum plasma concentration levels (Cmin), mean Cmax, and AUC were dose proportional following 200, 400, and 600 mg daily doses. Time-to-peak plasma concentrations were ∼3–5 h and steady-state plasma concentrations were attained after 6–10 days of EFV therapy.2,4
High-fat meals produce an increase in EFV bioavailability. In capsule form, administration of a single 600 mg dose of EFV with a high-fat/high-caloric meal resulted in a mean increase of 22% and 39% in AUC and Cmax respectively in comparison to the plasma exposures achieved when given under fasted conditions while the tablet form was associated with a 28% increase in mean AUC and a 79% increase in mean Cmax.2,4
EFV is highly protein-bound (∼99.5–99.75%) to human plasma proteins, mainly albumin.2,4 Only ∼0.5% remains available as free drug. In HIV-1-infected patients who received EFV 200–600 mg once daily for at least 1 month, cerebrospinal fluid concentrations ranged from 0.26% to 1.19% (mean 0.69%) of the corresponding plasma concentration.4,8–10 This proportion is approximately threefold higher than the free, non-protein-bound fraction of EFV in plasma.2,4
METABOLISM AND ELIMINATION
EFV is metabolized primarily by the hepatic cytochrome P450 isoenzymes CYP3A4 and CYP2B6 into hydroxylated, inactive metabolites. These metabolites undergo subsequent glucuronidation. EFV is an inducer of its own metabolism. Ten days of therapy with 200–400 mg of EFV daily resulted in a lower than expected accumulation of medication (22–42% lower) and a shorter terminal half-life, 40–55 h after multiple doses compared with the single-dose half-life of 52–76 h.2
Terminal elimination half-life is prolonged in patients with chronic liver disease. EFV is excreted principally in the feces, both as metabolites and unchanged drug. Approximately 14–34% of a radio-labeled dose of EFV was recovered in the urine (less than 1% as unchanged drug) and 16–61% of a radio-labeled dose was recovered (primarily as unchanged drug).4,11 The effect of renal impairment on EFV elimination has not been studied to date, but it is expected to be minimal because only 1% of EFV is excreted unchanged in urine.2,4
Plasma concentrations of EFV may persist at therapeutic levels for 2 weeks or more after discontinuation of EFV. Although the reported half-life is 40–55 h, concentrations were found in a small group of patients tested at 0, 4, 7, and 21 days after stopping EFV therapy.12 If drugs with a shorter half-life are stopped at the same time as EFV, this may be, in effect, EFV monotherapy for the patients. It may be advisable therefore to stop EFV at least 2 weeks before stopping other drugs to avoid the emergence of drug resistance.12,13
PHARMACOKINETICS, GENDER, ETHNICITY, AND RACE
It is unclear whether sex-determined pharmacokinetic differences exist. EFV clearance and volume of distribution appeared to be similar between men and women in some studies14 while others found that females had a 25% higher mean EFV AUC.15
Significant associations between weight and both drug clearance and distribution have been found even after adjusting for sex and concomitant medications–those who weighed less had slower EFV clearance.4,14
Particular caution should be paid to patients of African origin. In clinical studies, significant associations have been found between drug clearance of EFV and race. One study showed EFV clearance to be lower in blacks and Hispanics compared to non-Hispanic white patients. The white subjects had a 32% higher drug clearance.14 Plasma concentrations in five out of ten patients in another pharmacokinetic study had EFV elimination half-lives of longer than 100 h (114–229) although the T½ for EFV is 40–55 h. Four of these five were black African women. Three of these women showed therapeutic levels of greater than 1000 ng/mL 2 weeks after discontinuing EFV. The five patients with EFV half-lives within expected ranges were male Caucasians.16
It has been recently suggested that genetic polymorphisms are associated with differences in drug metabolism. EFV is primarily metabolized in the liver by the CYP450 2B6 enzyme and variations in this gene include the G/G, G/T, and the T/T genotypes. Haas and colleagues evaluated six allelic variants from patient DNA samples and found that the homozygous T/T genotype at position G516T was more prevalent in blacks (20%) than in Caucasians (3%), and was associated with greater EFV plasma exposure. The median EFV AUC drug exposure was threefold higher (130 μg h−1 L−1) with the T/T genotype compared with 44 and 60 μg h−1 L−1 in patients with G/G and G/T respectively. The T/T variant was also strongly associated with adverse central nervous system symptoms at week one.17,18
DRUG INTERACTIONS
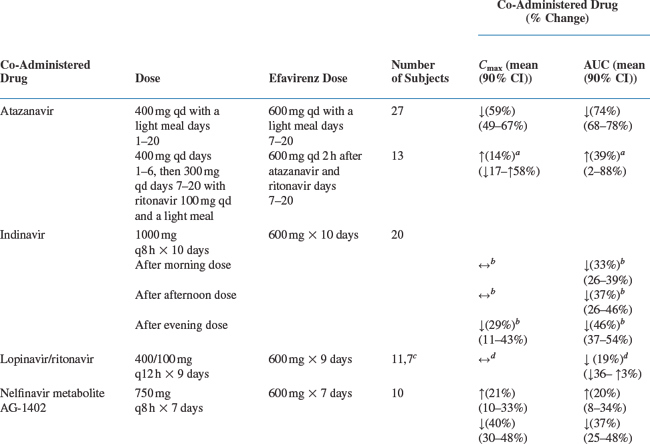
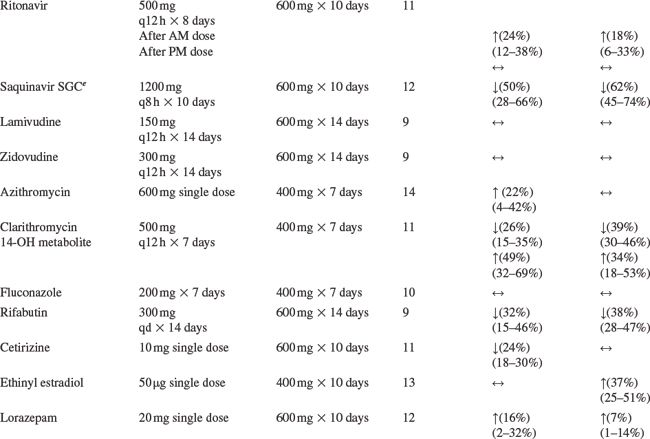
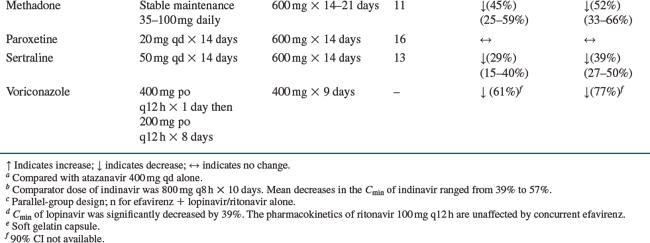
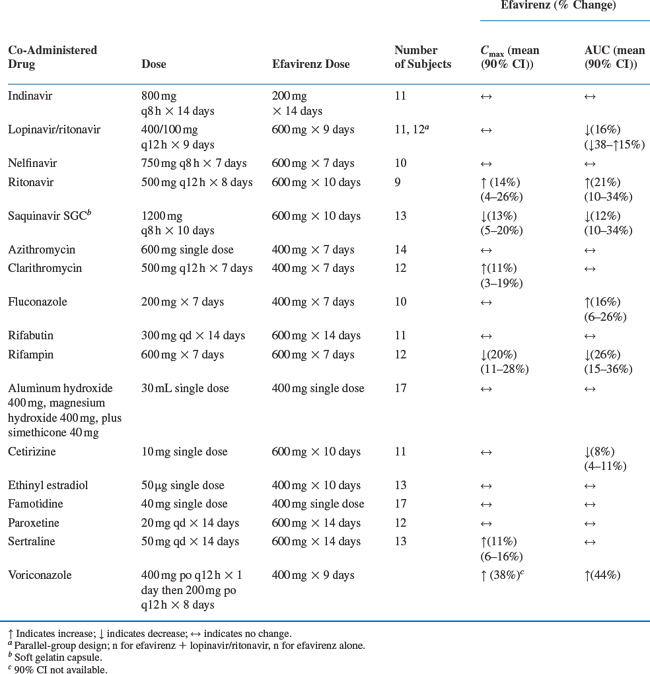
Drug interaction studies have been performed with EFV and other drugs likely to be co-administered and with drugs commonly used. The effects of EFV co-administration on other drugs–plasma AUC and Cmax–are shown in Table 14-1 and the effects of other drugs on EFV concentrations are shown in Table 14-2.4 As drug concentrations may be altered by co-administration with other drugs known to induce CYP3A4 activity, appropriate dose adjustments may be necessary (see section on Dosage).
A study of the effect of EFV investigated the effect of 600 mg/day EFV on the plasma pharmacokinetics of 40 mg/day SIM, 10 mg/day ATR and 40 mg PRA. Both healthy and HIV-infected patients were examined. Results showed that EFV reduced SIM AUC exposure by 58%, reduced ATR exposure by 43%, and decreased PRA concentrations by 40%. Neither SIM nor ATR nor PRA affected the non-steady-state EFV concentrations. Thus, the normal doses of SIM, ATR, and PRA may result in diminished antilipid efficacy when combined with EFV.19,20
The antituberculosis drug rifampicin decreases EFV concentrations levels and a common practice has been to increase the EFV dose to 800 mg/day.21 Caution must be taken, however, with patients of black African origin. Recent reports noted a very severe increase in EFV concentrations in some black African patients while taking both EFV and rifampicin causing significant clinical toxicity after EFV was increased to 800 mg/day.22,23
Dosage
The recommended dosage for adults and adolescents is 600 mg once daily, combined with a backbone of certain NRTIs or PIs. The recommended combination for initial therapy in treatment-naive adults and adolescents according to the 10 Oct 2006 Guidelines6 are: EFV with (lamivudine or emtricitabine) and (zidovudine or tenofovir DF) but not recommended for use in first trimester of pregnancy or in women with high pregnancy potential. The alternative regimens are: EFV with (lamivudine or emtricitabine) and (abacavir or didanosine).
Dose adjustments may be necessary when co-administering EFV with other PYP3A4 inducers. The manufacturer’s recommended dose changes, adjustments, and comments are listed in Table 14-3.4
Table 14-3 Established Drug Interactions
| Drug Name | Effect | Clinical Comment |
|---|---|---|
| Atazanavir | ↓ Atazanavir | When co-administered with Sustiva in treatment-naive patients, the recommended dose of atazanavir is 300 mg with ritonavir 100 mg and Sustiva 600 mg (all once daily). Dosing recommendations for Sustiva and atazanavir in treatment-experienced patients have not been established. |
| Clarithromycin | ↓ Clarithromycin concentration | Plasma concentrations decreased by Sustiva; clinical significance unknown. In uninfected volunteers, 46% developed rash while receiving Sustiva and clarithromycin. No dose adjustment of Sustiva is recommended when given with clarithromycin. Alternatives to clarithromycin, such as azithromycin, should be considered (see Other Drugs, following table). Other macrolide antibiotics, such as erythromycin, have not been studied in combination with Sustiva. |
| ↑ 14-OH metabolite concentration | ||
| Indinavir | ↓ Indinavir concentration | The optimal dose of indinavir, when given in combination with Sustiva, is not known. Increasing the indinavir dose to 1000 mg every 8 h does not compensate for the increased indinavir metabolism due to Sustiva. When indinavir at an increased dose (1000 mg every 8 h) was given with Sustiva (600 mg once daily), the indinavir AUC and Cmin were decreased on average by 33–46% and 39–57%, respectively, compared to when indinavir (800 mg every 8 h) was given alone. |
| Lopinavir/ritonavir | ↓ Lopinavir concentration | A dose increase of lopinavir/ritonavir to 533/133 mg (four capsules or 6.5 mL) twice daily taken with food is recommended when used in combination with Sustiva. |
| Methadone | ↓ Methadone concentration | Co-administration in HIV-infected individuals with a history of injection drug use resulted in decreased plasma levels of methadone and signs of opiate withdrawal. Methadone dose was increased by a mean of 22% to alleviate withdrawal symptoms. Patients should be monitored for signs of withdrawal and their methadone dose increased as required to alleviate withdrawal symptoms. |
| Ethinyl estradiol | ↑ Ethinyl estradiol concentration | Plasma concentrations increased by Sustiva; clinical significance unknown. Because the potential interaction of efavirenz with oral contraceptives has not been fully characterized, a reliable method of barrier contraception should be used in addition to oral contraceptives. |
| Rifabutin | ↓ Rifabutin concentration | Increase daily dose of rifabutin by 50%. Consider doubling the rifabutin dose in regimens where rifabutin is given two or three times a week. |
| Rifampin | ↓ Efavirenz concentration | Clinical significance of reduced efavirenz concentrations unknown. |
| Ritonavir | ↑ Ritonavir concentration | Combination was associated with a higher frequency of adverse clinical experiences (e.g., dizziness, nausea, paresthesia) and laboratory abnormalities (elevated liver enzymes). Monitoring of liver enzymes is recommended when Sustiva is used in combination with ritonavir. |
| ↓ Efavirenz concentration | ||
| Saquinavir | ↓ Saquinavir concentration | Should not be used as sole protease inhibitor in combination with Sustiva. |
| Sertraline | ↓ Sertraline concentration | Increases in sertraline dose should be guided by clinical response. |
| Other Potentially Clinically Significant Drug or Herbal Product Interactions with Sustivaa | ||
| Anticoagulants: Warfarin | Plasma concentrations and effects potentially increased or decreased by Sustiva. | |
| Anticonvulsants: Phenytoin | Potential for reduction in anticonvulsant and/or efavirenz plasma levels; periodic monitoring of anticonvulsant plasma levels should be conducted. | |
| Phenobarbital | ||
| Carbamazepine | ||
| Antifungals: Itraconazole | Drug interaction studies with Sustiva and these imidazole and triazole antifungals have not been conducted. Sustiva has the potential to decrease plasma concentrations of itraconazole and ketoconazole. | |
| Ketoconazole | ||
| Anti-HIV protease inhibitors: | No pharmacokinetic data are available. | |
| Saquinavir/ritonavir combination | Sustiva has the potential to decrease serum concentrations of amprenavir | |
| Amprenavir. | ||
| NNRTIs | No studies have been performed with other NNRTIs. | |
| St John’s wort (Hypericum perforatum) | Expected to substantially decrease plasma levels of efavirenz; has not been studied in combination with Sustiva. | |
| Drugs That Should Not Be Co-administered with Sustiva | ||
| Antihistamines | Astemizole | |
| Benzodiazepines | Midazolam, triazolam | |
| GI motility agents | Cisapride | |
| Antimigraine | Ergot derivatives | |
| Antifungal | Voriconazole | |
Pediatric Dosing
The recommended dose for children weighing less than 88 lbs depends on the child’s weight (see Table 14-4). For children (or adults) who are not able to swallow capsules, a liquid formulation of EFV is available.4,5,24
| Pediatric Dose to Be Administered Once Daily | ||
|---|---|---|
| Body Weight | ||
| kg | lbs | Sustiva Dose (mg) |
| 10 to <15 | 22 to <33 | 200 |
| 15 to <20 | 33 to <44 | 250 |
| 20 to <25 | 44 to <55 | 300 |
| 25 to <32.5 | 55 to <71.5 | 350 |
| 32.5 to <40 | 71.5 to <88 | 400 |
| ≥40 | ≥88 | 600 |
TOXICITY
The most common adverse events noted during EFV therapy are rash and central nervous system symptoms.4,25,26 Rash generally occurs at a median 9 days after start of EFV therapy. Twenty-six percent of EFV-treated patients compared to 17% in the control arms experienced rash in controlled clinical trials. Grade 4 rash occurred only in 0.1% of patients. The rash is usually self-limiting in ∼16 days. EFV should be discontinued if blistering, ulceration or desquamation occurs. Rash was reported in 46% (26/57) of pediatric patients and 2/26 experienced grade 4 rash (see section on Pediatric Toxicities).
Fifty-three percent of patients receiving EFV in controlled trials reported central nervous system symptoms compared to 25% of patients receiving control regimens. These symptoms included, but were not limited to, dizziness (28.1%), insomnia (16.3%), impaired concentration (8.3%), somnolence (7.0%), abnormal dreams (6.2%), and hallucinations (1.2%). These symptoms were severe in 2.0% of patients and 2.1% of patients discontinued therapy as a result. These symptoms usually begin during the first or second day of therapy and generally resolve after the first 2–4 weeks of therapy. After 4 weeks of therapy, the prevalence of nervous system symptoms of at least moderate severity ranged from 5% to 9% in patients treated with regimens containing EFV and from 3% to 5% in patients treated with a control regimen.4,26
The symptoms generally improve with continued therapy and are not predictive of subsequent onset of the less frequent psychiatric symptoms. Dosing at bedtime may improve the tolerability of these nervous system symptoms. Analysis of long-term data from Study 006 for patients treated with either: EFV with zidovudine and lamivudine, or EFV with indinavir, or with indinavir and zidovudine + lamivudine, showed that beyond 24 weeks of therapy the incidences of new-onset nervous system symptoms among EFV-treated patients were generally similar to those in the indinavir-containing control arm.4
Psychiatric Symptoms
Serious psychiatric adverse experiences have been reported in patients treated with EFV. In controlled trials of 1008 patients treated with regimens containing EFV for a mean of 2.1 years and 635 patients treated with control regimens for a mean of 1.5 years, the frequency of specific serious psychiatric events among patients who received EFV or control regimens, respectively, were: severe depression (2.4%, 0.9%), suicidal ideation (0.7%, 0.3%), nonfatal suicide attempts (0.5%, 0%), aggressive behavior (0.4%, 0.5%), paranoid reactions (0.4%, 0.3%), and manic reactions (0.2%, 0.3%). When psychiatric symptoms similar to those noted above were combined and evaluated as a group in a multivariable analysis of data from Study 006, treatment with EFV was associated with an increase in the occurrence of these selected psychiatric symptoms. Other factors associated with an increase in the occurrence of these psychiatric symptoms were history of injection drug use, psychiatric history, and receipt of psychiatric medication at study entry; similar associations were observed in both the EFV and control groups. In Study 006, onset of new serious psychiatric symptoms occurred throughout the study for both EFV-treated and control-treated patients. One percent of EFV-treated patients discontinued or interrupted treatment because of one or more of these selected psychiatric symptoms. There have also been occasional postmarketing reports of death by suicide, delusions, and psychosis-like behavior; although a causal relationship to the use of EFV cannot be determined from these reports. Patients with serious psychiatric adverse experiences should seek immediate medical evaluation to assess the possibility that the symptoms may be related to the use of EFV, and if so, to determine whether the risks of continued therapy outweigh the benefits.4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree