Early-Stage Invasive Breast Cancer | 5 |
Paula Rosenblatt, J. W. Snider III, Steven J. Feigenberg, Neha Bhooshan, Sally B. Cheston, Susan B. Kesmodel, Lindsay Hessler, Julia Terhune, Olga Ioffe, Rachel White, Yang Zhang, Ina Lee, Krista Chain, Chad Tarabolous, Rawand Faramand, Angela DeRidder, Jennifer Ding, and Katherine H. R. Tkaczuk
EPIDEMIOLOGY/GENERAL CHARACTERISTICS
In the United States, one in eight women will develop breast cancer (BC) in their lifetime. In 2015, 231,840 new cases of invasive breast carcinoma were diagnosed, representing 14% of all new cancer cases (1). While rates for new BC cases have been stable over the last 10 years, death rates have been falling on average 1.9% each year. Still, approximately 40,000 BC-related deaths occur in the United States annually (1). Early-stage presentation is most common in this country due to screening, with 61% of women presenting with localized (confined to the primary site) BC and an additional 32% with regional (spread to regional lymph nodes) BC (1). Five-year survival is excellent following standard treatments for patients with localized (98.6%) and regional (84.9%) involvement at presentation (1). With more extensive local–regional involvement, such as skin, chest wall, or internal mammary and supraclavicular lymph nodes, outcomes are significantly worse.
Risk Factors for Breast Cancer
Risks include personal history of breast disease, family history of BC, hormonal exposures, and life exposures. Specifics are contained in Box 5.1.
Symptoms and Signs
In the United States nearly 60% of patients with early-stage BC present due to an abnormal screening mammogram. The other 40% present with a palpable breast mass, change in breast contour, or nipple discharge (26,27). Only 2% to 7% of patients with a malignant mass initially present with breast pain (28). Physical exam findings concerning for malignancy include: a hard, irregular, or nodular breast lump; skin tethering; nipple inversion; dilated veins; ulceration; peau d’orange; and nipple changes (inversion or excoriation). Metastatic BC can spread to the bones, liver, lungs, brain, and other organs (29). The route of spread for BC is via local lymphatic channels and hematogenous. A thorough history and physical exam looking for signs and symptoms of metastatic disease is warranted in anyone with a concerning breast mass.
Box 5.1 Risks for Breast Cancer Include Both Modifiable and Nonmodifiable Factors
Nonmodifiable Factors That Increase Risk of Breast Cancer
• Known genetic mutation (for details, see Chapter 9)
• Gender—100:1 female:male incidence BC (1)
• Age—age <50, risk 1:53; age >70 to death, risk 1:15 (1)
• Personal history of breast disease
![]() Dense breast—4–5 × risk BC vs. women with less dense tissue (2,3)
Dense breast—4–5 × risk BC vs. women with less dense tissue (2,3)
![]() Atypical ductal hyperplasia—up to 30% risk of BC at 25 years (4)
Atypical ductal hyperplasia—up to 30% risk of BC at 25 years (4)
![]() LCIS—1% risk of invasive disease per year; RR 2–8 depending on study (5–7)
LCIS—1% risk of invasive disease per year; RR 2–8 depending on study (5–7)
![]() DCIS—at 20 years, 6.2% risk of invasive disease, higher with younger age at diagnosis (8)
DCIS—at 20 years, 6.2% risk of invasive disease, higher with younger age at diagnosis (8)
![]() Invasive BC—4% risk of contralateral BC at 7.5 years (9)
Invasive BC—4% risk of contralateral BC at 7.5 years (9)
• Family history
![]() Age of first degree relative diagnosis—RR 2.1 for mother diagnosed before age 40 (95% CIs [1.6, 2.8]) as opposed to 1.5 after age 70 (95% CIs [1.1, 2.2]) (10)
Age of first degree relative diagnosis—RR 2.1 for mother diagnosed before age 40 (95% CIs [1.6, 2.8]) as opposed to 1.5 after age 70 (95% CIs [1.1, 2.2]) (10)
![]() Number of first degree relatives—the risk ratios were 1.80, 2.93, and 3.90, respectively, for one, two, and three or more affected first degree relatives (P < .0001 each) (11)
Number of first degree relatives—the risk ratios were 1.80, 2.93, and 3.90, respectively, for one, two, and three or more affected first degree relatives (P < .0001 each) (11)
• Hormonal factors
![]() Age of menarche—menarche at age ≥15 vs. <13%–24% decreased ER/PR+ BC and 16% decreased HR− BC (HR 0.76, 95% CIs [0.68, 0.85] and HR 0.84, CIs [0.69, 1.03]) (12)
Age of menarche—menarche at age ≥15 vs. <13%–24% decreased ER/PR+ BC and 16% decreased HR− BC (HR 0.76, 95% CIs [0.68, 0.85] and HR 0.84, CIs [0.69, 1.03]) (12)
![]() Null parity and increasing age at childbirth associated with increased risk—cumulative incidence up to age 70 years was about 20% lower, 10% lower, or 5% higher for parous vs. nulliparous women if their first birth was at age 20, 25, or 35 years, respectively (13,14)
Null parity and increasing age at childbirth associated with increased risk—cumulative incidence up to age 70 years was about 20% lower, 10% lower, or 5% higher for parous vs. nulliparous women if their first birth was at age 20, 25, or 35 years, respectively (13,14)
Modifiable—Lifestyle Risk Factors That Increase BC Risk
• Postmenopausal long hormone replacement therapy term (>3 y) (15)
• Alcohol consumption—dose response starting at three drinks per week: RR 1.15 (95% CIs [1.06, 1.24]) (16)
• Weight gain—NHS gains of 25.0 kg since age 18: RR 1.45 (95% CIs [1.27, 1.66]; P < .001) and 10 kg since menopause: RR 1.18 (95% CIs [1.03, 1.35]; P = .002) (17)
• IGF-1 and endogenous insulin levels—IGF-1 concentration of highest versus the lowest fifth OR 1.28 (95% CIs [1.14, 1.44]; P < .0001) (18,19)
• Postmenopausal obesity although premenopausal obesity NOT associated (20)
• Chest radiation exposure—highest risk with adolescence exposure: RR 15–25; each Gray unit received by any breast increased the excess relative risk of BC by 0.13 (21,22)
Modifiable Risk Factors That Decrease BC Risk
• Exercise—regular physical exercise reduced the risk of BC by 25%, especially among postmenopausal women: reduced serum estrogens, insulin, and IGF-1 levels (23)
• Breastfeeding—for every 12 months of breastfeeding, there was a 4.3% reduction in the relative risk of BC although data confounded by parity (24,25)
BC, breast cancer; DCIS, ductal carcinoma in situ; ER/PR, hormone receptors, estrogen-ER, progesterone-PR; HR, hazard ratio; IGF-1, insulin growth factor-1; LCIS, lobular carcinoma in situ; NHS, Nurses’ Health Study; RR, relative risk.
Diagnosis and Workup of Breast Cancer
As discussed in Chapter 1, the majority of patients can be diagnosed utilizing nonsurgical image-guided breast and axillary biopsies. In most cases, image-guided core needle biopsy (CNB) provides tissue to confirm the diagnosis of noninvasive or invasive BC and to obtain tissue markers, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2)/neu expression, and Ki-67 by immunohistochemical (IHC) stains.
 We recommend that all patients with clinically or mammographically suspicious breast abnormalities be assessed with nonsurgical core needle biopsy approaches first before surgical excision for diagnosis.
We recommend that all patients with clinically or mammographically suspicious breast abnormalities be assessed with nonsurgical core needle biopsy approaches first before surgical excision for diagnosis.
Multidisciplinary evaluation of every patient at the time of diagnosis is standard. We review the pathology slides and imaging studies with a team, which includes specialists in breast imaging, pathology, breast surgical oncology, radiation oncology, medical oncology, and genetic counseling. Our nurse navigator, research nurses, and nurse coordinators are also present during our multidisciplinary conference and clinic to assist the patient and providers and ensure seamless and comprehensive care.
Pretreatment workup should include a full history and physical examination with discussion of symptoms (bone pains, breathing problems, nausea, abdominal pains, and headaches) and bilateral breast exam and examination of the regional nodal basins (axillary, infraclavicular, supraclavicular, and cervical).
Initial laboratory tests include a complete blood count (CBC) and comprehensive metabolic panel (CMP), which include liver function and alkaline phosphatase.
Local/regional staging: We recommend additional imaging of the axilla and biopsy of abnormal lymph nodes. This approach is helpful in determining the extent and type of axillary surgery. Women who have biopsy-proven positive axillary lymph nodes are usually recommended a level 1 and 2 lymph node dissection as opposed to a sentinel lymph node biopsy (SLNB).
Systemic imaging of clinically asymptomatic patients with presumed stage 1 and 2 disease is NOT necessary and we recommend against routine systemic imaging (30). In our practice, patients with stage 3 BC undergo staging with CT of chest/abdomen/pelvis with intravenous contrast and bone scan or a fludeoxyglucose (FDG) PET/CT to rule out distant metastasis. Additional systemic imaging is considered based on clinical symptoms. For instance, if new headaches or neurologic symptoms are present, a contrast-enhanced CT or MRI of the brain should be performed to rule out intracranial involvement. In patients with new back pain and/or unilateral leg weakness or urinary/fecal incontinence, additional contrast-enhanced imaging (preferably MRI) of the entire spine should be done to rule out spinal cord involvement.
Psychosocial assessment of patients’ comorbidities, social situation, family support, financial concerns, and ability to understand the risks and benefits of the treatment should be assessed at this initial evaluation. These variables should factor into the recommended surgery and adjuvant treatments. We encourage family and friends to be present during our multidisciplinary consultations. In addition, we work closely with American Cancer Society (ACS) certified patient advocates who often sit in on the consultations and help patients to understand the proposed treatments.
General Staging
Currently, staging is based on the seventh edition of the American Joint Commission on Cancer (AJCC) Tumor, Nodes, Metastasis (TNM) staging system (Tables 5.1 and 5.2).
Clinical staging includes tumor size and lymph node assessment by clinical breast exam and breast/systemic imaging.
Pathological staging is the assessment of tumor size and locoregional lymph node involvement by the pathologist after breast surgery is completed.
Neoadjuvant systemic therapy staging: Patients who are offered systemic treatment before surgery will have clinical staging prior to therapy and pathologic response after neoadjuvant therapy, designated as ypTNM.
AJCC Staging—TNM
Table 5.1 TNM Classification for Breast Cancer | |
Primary tumor (T) | |
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | Carcinoma in situ |
Tis (DCIS) | Ductal carcinoma in situ |
Tis (LCIS) | Lobular carcinoma in situ |
Tis (Paget) | Paget disease of the nipple NOT associated with invasive carcinoma and/or carcinoma in situ (DCIS and/or LCIS) in the underlying breast parenchyma. Carcinomas in the breast parenchyma associated with Paget disease are categorized based on the size and characteristics of the parenchymal disease, although the presence of Paget disease should still be noted |
T1 | Tumor ≤20 mm in greatest dimension |
T1mi | Tumor ≤1 mm in greatest dimension |
T1a | Tumor >1 mm but ≤5 mm in greatest dimension |
T1b | Tumor >5 mm but ≤10 mm in greatest dimension |
T1c | Tumor >10 mm but ≤20 mm in greatest dimension |
T2 | Tumor >20 mm but ≤50 mm in greatest dimension |
T3 | Tumor >50 mm in greatest dimension |
T4 | Tumor of any size with direct extension to the chest wall and/or to the skin (ulceration or skin nodules) |
T4a | Extension to chest wall, not including only pectoralis muscle adherence/invasion |
T4b | Ulceration and/or ipsilateral satellite nodules and/or edema (including peau d’orange) of the skin, which do not meet the criteria for inflammatory carcinoma |
T4c | Both T4a and T4b |
T4d | Inflammatory carcinoma |
Regional lymph nodes (N) | |
Clinical | |
NX | Regional lymph nodes cannot be assessed (eg, previously removed) |
N0 | No regional lymph node metastasis |
N1 | Metastasis to movable ipsilateral level I, II axillary lymph node(s) |
N2 | Metastases in ipsilateral level I, II axillary lymph nodes that are clinically fixed or matted or in clinically detected* ipsilateral internal mammary nodes in the absence of clinically evident axillary lymph node metastasis |
N2a | Metastases in ipsilateral level I, II axillary lymph nodes fixed to one another (matted) or to other structures |
N2b | Metastases only in clinically detected* ipsilateral internal mammary nodes and in the absence of clinically evident level I, II axillary lymph node metastases |
N3 | Metastases in ipsilateral infraclavicular (level III axillary) lymph node(s), with or without level I, II axillary node involvement, or in clinically detected* ipsilateral internal mammary lymph node(s) and in the presence of clinically evident level I, II axillary lymph node metastasis; or metastasis in ipsilateral supraclavicular lymph node(s), with or without axillary or internal mammary lymph node involvement |
N3a | Metastasis in ipsilateral infraclavicular lymph node(s) |
N3b | Metastasis in ipsilateral internal mammary lymph node(s) and axillary lymph node(s) |
N3c | Metastasis in ipsilateral supraclavicular lymph node(s) |
*“Clinically detected” is defined as detected by imaging studies (excluding lymphoscintigraphy) or by clinical examination and having characteristics highly suspicious for malignancy or a presumed pathologic macrometastasis on the basis of fine needle aspiration (FNA) biopsy with cytologic examination. | |
Pathologic (pN)* | |
pNX | Regional lymph nodes cannot be assessed (eg, previously removed, or not removed for pathologic study) |
pN0 | No regional lymph node metastasis identified histologically. Note: Isolated tumor cell clusters (ITCs) are defined as small clusters of cells ≤0.2 mm, or single tumor cells, or a cluster of <200 cells in a single histologic cross-section; ITCs may be detected by routine histology or by immunohistochemical (IHC) methods; nodes containing only ITCs are excluded from the total positive node count for purposes of N classification but should be included in the total number of nodes evaluated |
pN0(i−) | No regional lymph node metastases histologically, negative IHC |
pN0(i+) | Malignant cells in regional lymph node(s) ≤0.2 mm (detected by hematoxylin–eosin [H&E] stain or IHC, including ITC) |
pN0(mol−) | No regional lymph node metastases histologically, negative molecular findings (reverse transcriptase polymerase chain reaction [RT-PCR]) |
pN0(mol+) | Positive molecular findings (RT-PCR) but no regional lymph node metastases detected by histology or IHC |
pN1 | Micrometastases; or metastases in 1–3 axillary lymph nodes and/or in internal mammary nodes, with metastases detected by sentinel lymph node biopsy but not clinically detected† |
pN1mi | Micrometastases (>0.2 mm and/or >200 cells, but none >2.0 mm) |
pN1a | Metastases in 1–3 axillary lymph nodes (at least 1 metastasis >2.0 mm) |
pN1b | Metastases in internal mammary nodes, with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected† |
pN1c | Metastases in 1–3 axillary lymph nodes and in internal mammary lymph nodes, with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected† |
pN2 | Metastases in 4–9 axillary lymph nodes or in clinically detected‡ internal mammary lymph nodes in the absence of axillary lymph node metastases |
pN2a | Metastases in 4–9 axillary lymph nodes (at least 1 tumor deposit >2.0 mm) |
pN2b | Metastases in clinically detected‡ internal mammary lymph nodes in the absence of axillary lymph node metastases |
pN3 | Metastases in ≥10 axillary lymph nodes; or in infraclavicular (level III axillary) lymph nodes; or in clinically detected‡ ipsilateral internal mammary lymph nodes in the presence of ≥1 positive level I, II axillary lymph nodes; or in >3 axillary lymph nodes and in internal mammary lymph nodes, with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected†; or in ipsilateral supraclavicular lymph nodes |
pN3a | Metastases in ≥10 axillary lymph nodes (at least 1 tumor deposit >2.0 mm); or metastases to the infraclavicular (level III axillary lymph) nodes |
pN3b | Metastases in clinically detected‡ ipsilateral internal mammary lymph nodes in the presence of ≥1 positive axillary lymph nodes; or in >3 axillary lymph nodes and in internal mammary lymph nodes, with micrometastases or macrometastases detected by sentinel lymph node biopsy but not clinically detected† |
pN3c | Metastases in ipsilateral supraclavicular lymph nodes |
*Classification is based on axillary lymph node dissection, with or without sentinel lymph node biopsy. Classification based solely on sentinel lymph node biopsy without subsequent axillary lymph node dissection is designated (sn) for “sentinel node”—for example, pN0(sn). †“Not clinically detected” is defined as not detected by imaging studies (excluding lymphoscintigraphy) or not detected by clinical examination. ‡“Clinically detected” is defined as detected by imaging studies (excluding lymphoscintigraphy) or by clinical examination and having characteristics highly suspicious for malignancy or a presumed pathologic macrometastasis on the basis of FNA biopsy with cytologic examination. | |
Distant metastasis (M) | |
M0 | No clinical or radiographic evidence of distant metastasis |
cM0(i+) | No clinical or radiographic evidence of distant metastases, but deposits of molecularly or microscopically detected tumor cells in circulating blood, bone marrow, or other nonregional nodal tissue that are no larger than 0.2 mm in a patient without symptoms or signs of metastases |
M1 | Distant detectable metastases as determined by classic clinical and radiographic means and/or histologically proven >0.2 mm |
TNM, tumor, nodes, metastasis. Source: Used with permission of the American Joint Committee on Cancer (AJCC), Chicago, Illinois. From Ref. (31). Edge S, Byrd DR, Compton CC, et al. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer Science + Business Media; 2010. | |
PATHOLOGY
Invasive breast carcinomas are the most common type of breast malignancy. The pathologic evaluation of these tumors includes the determination of multiple important prognostic and predictive factors. Most powerful among these are pathologic tumor stage (tumor size, lymph node status) (32,33), histologic type, histologic grade, lymphatic vascular space invasion, margin status, and the results of prognostic/predictive marker analysis.
Pathologic Tumor Stage
Pathologic staging is the gold standard and consists of tumor–lymph node–metastasis (TNM) components. Tumor size (T) is measured both grossly and microscopically; microscopic tumor size is most accurate. T1 tumors are ≤20 mm, T2 tumors are >20 mm but ≤50 mm, and T3 tumors are >50 mm in greatest dimension. T4 tumor stage is reserved for tumors of any size that exhibit direct extension into the chest wall and/or skin. Staging of tumors is extremely important for prognosis, and studies have shown a direct relationship between the size of breast tumors, the frequency of axillary nodal involvement, and patient survival (32).
Nodal status is a powerful prognostic factor (33). Nodal stage (N) increases with the number of involved axillary lymph nodes. A tumor is stage N1a when 1 to 3 axillary lymph nodes are involved, N2a when 4 to 9 axillary lymph nodes are involved, and N3a when 10 or more axillary lymph nodes are involved. Isolated tumor cells, which are malignant cells in regional lymph node(s) ≤0.2 mm, are referred to a N0 (i+). Micrometastasis are 0.2 mm to 2 mm and are designated as N1mic (Table 5.1). Isolated tumor cells in lymph nodes behave similar to node-negative cancers (34,35).
Histologic Types of Invasive Breast Carcinoma
BC, like many other malignancies, is often viewed as a single pathological entity, but in fact, this disease encompasses numerous histologic types. The histologic type imparts unique features to BC and often determines its behavior and impacts management. According to the World Health Organization (WHO) classification, there are up to 21 distinct histological types on the basis of cell morphology, growth, and architecture pattern. The most common and clinically significant histologic types of BC are invasive ductal carcinoma (IDC), lobular carcinoma, medullary carcinoma, mucinous carcinoma, tubular carcinoma, micropapillary carcinoma, and metaplastic carcinoma.
Invasive Ductal Carcinoma
IDC, sometimes referred to as invasive carcinoma of no special type, comprises 70% to 80% of breast carcinomas (Figure 5.1). If an IDC is well differentiated (histologic grade 1), the cells resemble cells lining normal breast ducts and lobules. These tumors typically form cohesive cell nests, which can mimic the architecture of normal breast tissue, forming glands and tubules. Typical gross appearance of IDC is that of a stellate firm mass with a fibrotic center. When IDC metastasize, they spread most frequently to the lymph nodes, liver, and central nervous system (36).
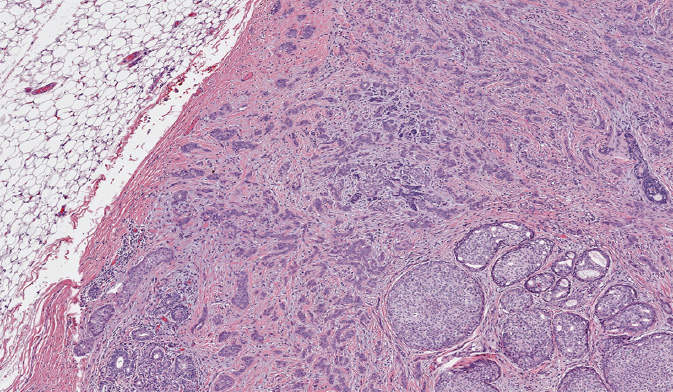
Figure 5.1 Invasive ductal carcinoma associated with ductal carcinoma in situ (lower right).
Invasive Lobular Carcinoma (ILC)
Invasive lobular carcinoma (ILC) accounts for approximately 10% of BC. Despite the implication of their name, lobular carcinomas arise from the same terminal duct/lobular cells as IDCs (Figure 5.2). The defining characteristic of lobular carcinomas is their loss of expression of intercellular adhesion protein E-cadherin (Figure 5.3), which can be confirmed with the aid of IHC stain. Although the prognosis of this histological type is similar to that of IDC, its clinicopathologic features differ considerably. Unlike IDC, lobular carcinoma cells infiltrate the surrounding tissue diffusely, by single cells, files, or in sheets (37). This diffuse pattern of infiltration makes early detection more difficult, as the cancer may not be visible on imaging or palpable on physical exam (38). Lobular carcinomas are more frequently multifocal (MF) and bilateral. MRI of the breast will often be performed to fully evaluate the extent of ILC given its underestimation on mammography. ILC may be more challenging to remove by breast conserving surgery (BCS) as it is often more extensive than expected. ILC will more frequently have positive surgical margins than ductal carcinomas (39). In addition, lobular carcinomas are more likely than ductal carcinomas to spread to the gastrointestinal organs, gynecologic organs, peritoneum, and meninges (36).
Pleomorphic lobular carcinoma is a very rare and aggressive variant of lobular carcinoma (accounting for <1% of all invasive mammary carcinomas) that frequently presents with lymphovascular invasion and at advanced stage (40). The growth pattern of this tumor is identical to that seen in classical lobular carcinoma but the pleomorphic variant is composed of cells with more evidence of nuclear atypia and pleomorphism. HER2 is overexpressed in up to 30% of the cases of pleomorphic lobular carcinoma metastasis (40).
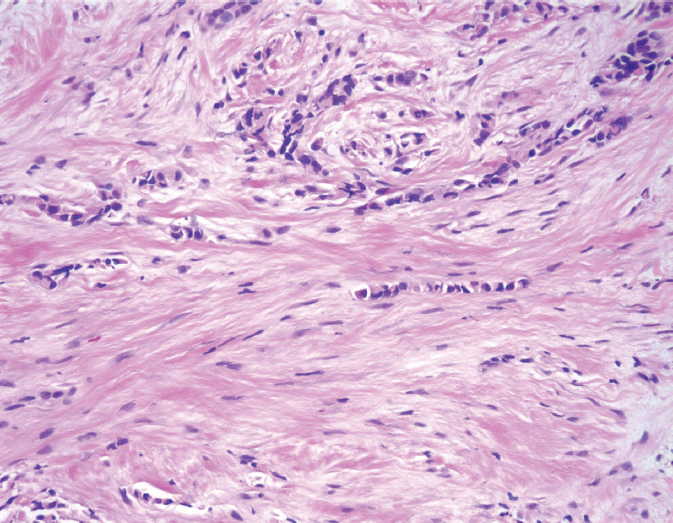
Figure 5.2 Invasive lobular carcinoma. The tumor cells are very small and bland, and infiltrate as single cells and cell files.
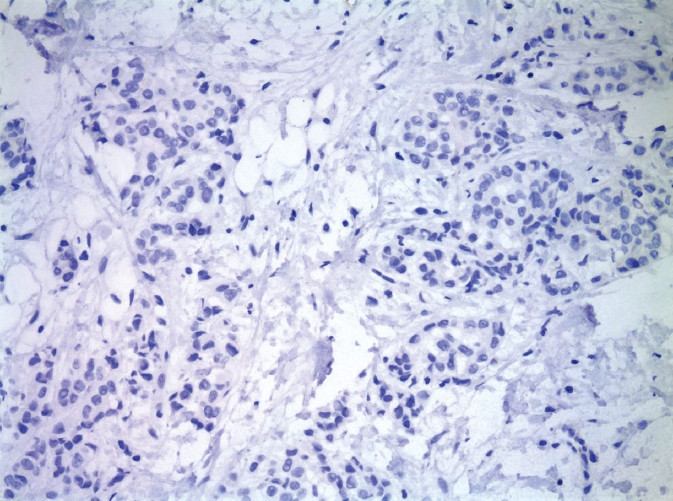
Figure 5.3 Invasive lobular carcinoma, negative E-cadherin immunostain.
Other Histological Types
Medullary carcinomas account for 1% to 7% of BC. The tumors usually present as a discrete lobulated mass (41). The tumors are well circumscribed and exhibit overtly malignant morphology and high degree of cytologic atypia and mitotic activity. The histologic grade of these tumors is always high. Notably, medullary carcinomas are often infiltrated with immune cells, such as lymphocytes and plasma cells. These features must be present in the entire tumor for the diagnosis of classical medullary carcinoma. Cases that do not fulfill all these criteria are defined as atypical medullary carcinoma or carcinoma with medullary features. Medullary carcinomas are usually triple negative and have high Ki-67 index but are less likely to spread to the lymph nodes. Patients with medullary carcinoma have a more favorable prognosis compared to those with ductal or lobular carcinoma (42). An association of medullary carcinomas with BRCA1 mutations has been reported.
Mucinous carcinomas, tubular and cribriform carcinomas account for a small percentage of well-differentiated BCs. Mucinous carcinomas occur most frequently in older patients and are low grade, with clusters of bland cells floating in lakes of mucin (Figure 5.4). Tubular and cribriform carcinomas more often affect younger women and can be MF. The tumor cells are very bland and form angulated tubules or cribriform, sieve-like nests. Mucinous, cribriform, and tubular carcinomas have a favorable prognosis as compared to the other histologic types of BC; they typically are low grade, strongly express ER and PR, are negative for HER2, and have a low proliferation rate (43).
Invasive micropapillary carcinoma is a tumor with a peculiar appearance characterized by rounded tumor nests surrounded by empty spaces (Figure 5.5). These tumors tend to be very aggressive, with massive lymph node metastases and extensive lymphatic vascular space invasion resulting in intramammary tumor spread with MF and multicentric (MC) tumors (44).
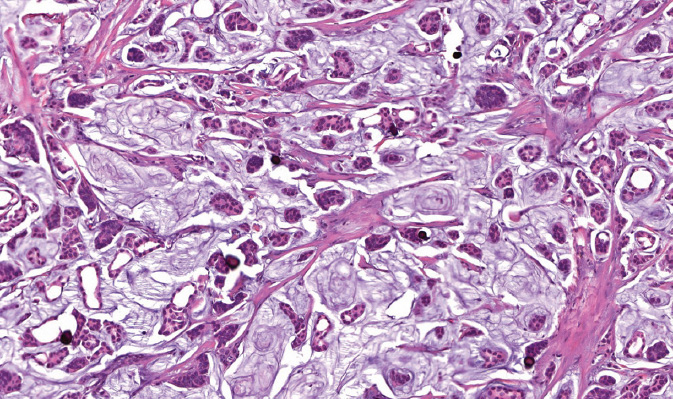
Figure 5.4 Invasive mucinous (colloid) carcinoma. Neoplastic nests of cells floating in lakes of extravasated mucin.
Metaplastic carcinomas are a heterogeneous group of tumors characterized by aberrant differentiation of the neoplastic epithelium into squamous or mesenchymal phenotype. The WHO classifies these as squamous cell carcinoma, spindle cell carcinoma, and metaplastic carcinoma with mesenchymal differentiation (also called matrix-producing carcinomas). These tumors are high grade, triple negative, and have a high Ki-67 index. The metaplastic carcinoma group also includes low-grade adenosquamous carcinomas and fibromatosis-like metaplastic carcinomas that are associated with a more favorable prognosis.
Histologic Grade
The most widely utilized histologic grading system for invasive carcinomas of the breast is the Nottingham combined histologic grade (Table 5.3). The variables measured in this system include the degree of glandular/tubular differentiation, the extent of nuclear atypia, and the mitotic rate (number of mitotic figures per 10 high-power fields). Each of the three parameters is scored on a scale of 1 to 3, and the final Nottingham grade (Table 5.3) is the sum of these three scores. All invasive carcinomas of the breast, regardless of histologic type (Figure 5.6), should be graded, as histologic grade has been shown to be strongly associated with overall survival (OS) (45). Figure 5.6 illustrates an example of a high grade tumor.
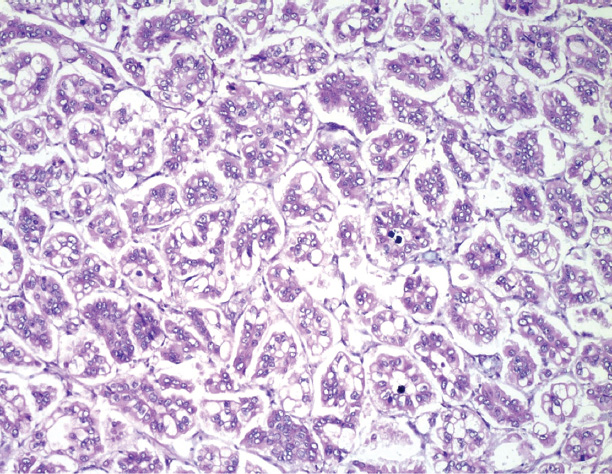
Figure 5.5 Invasive micropapillary carcinoma. Tumor cell nests grow as small papillary clusters surrounded by retraction artifact.
Table 5.3 Nottingham Combined Histologic Grade |
Glandular/tubular differentiation |
Score 1: >75% of tumor area forming glandular/tubular structures |
Score 2: 10%–75% of tumor area forming glandular/tubular structures |
Score 3: <10% of tumor area forming glandular/tubular structures |
Nuclear atypia |
Score 1: tumor nuclei similar to normal breast epithelial cell nuclei |
Score 2: intermediate nuclei with moderate variability in size and shape, visible nucleoli |
Score 3: large nuclei with marked variation in size and shape, prominent nucleoli |
Mitotic rate (per 10 HPF with 40 × objective and field area of 0.196 mm2) |
Score 1: 0–7 mitoses |
Score 2: 8–14 mitoses |
Score 3: 15 or more mitoses |
Overall grade |
Grade 1 (well differentiated): total score of 3, 4, or 5 |
Grade 2 (moderately differentiated): total score of 6 or 7 |
Grade 3 (poorly differentiated): total score of 8 or 9 |
HPF, high powered fields. |
Prognostic Markers
Over the last few decades, the use of immunophenotyping to stratify patients for prognostication and treatment selection has become the mainstay of workup of all newly diagnosed BC. The basic prognostic/predictive panel for invasive carcinomas of the breast includes IHC stains for ER, PR, HER2, and Ki-67 proliferative index.
ER/PR: Approximately 75% of all invasive breast carcinomas are positive for hormone receptors (HRs), with slightly more tumors being ER-positive than PR-positive. While ER and PR are weak favorable prognostic factors, they serve as strong predictive factors for how well a tumor will respond to hormonal therapy (46). Guidelines published by the College of American Pathologists (CAP) in 2010 state that BC demonstrating ER or PR immunoreactivity in at least 1% of tumor cells should be classified as receptor-positive (Figure 5.7) (47).
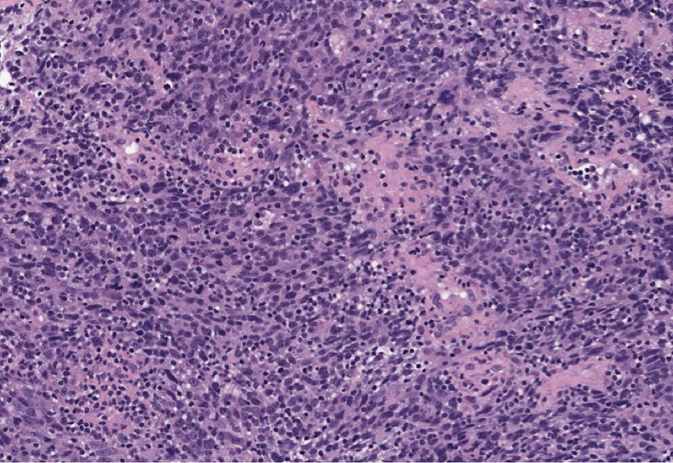
Figure 5.6 Basal-like invasive carcinoma. The tumor is high grade, grows as solid sheets, and is associated with lymphoid infiltrate.
HER2, also known as ERB-B2, is a signaling molecule that is upregulated in approximately 20% of invasive BC, and confers a worse prognosis. In addition, it is important to detect amplification of HER2 gene/overexpression of HER2 protein in BC (Figure 5.8) in order to identify patients who will benefit from anti-HER2 therapy. Immunohistochemistry and fluorescence in situ hybridization (FISH) are methods to detect HER2 overexpression. According to American Society of Clinical Oncology (ASCO)/CAP guidelines, HER2 status should be determined in all patients with invasive BC on the basis of one or more HER2 test results (negative, equivocal, or positive); if the initial HER2 test is equivocal, reflex testing should be performed on the same specimen using the alternative test or on an alternative specimen. Patients who were previously HER2-negative in the primary tumor and present with recurrent disease should have the metastatic site retested for HER2.

Figure 5.7 Estrogen receptor (ER) immunostain: most (>90%) of the tumor cells are strongly positive.
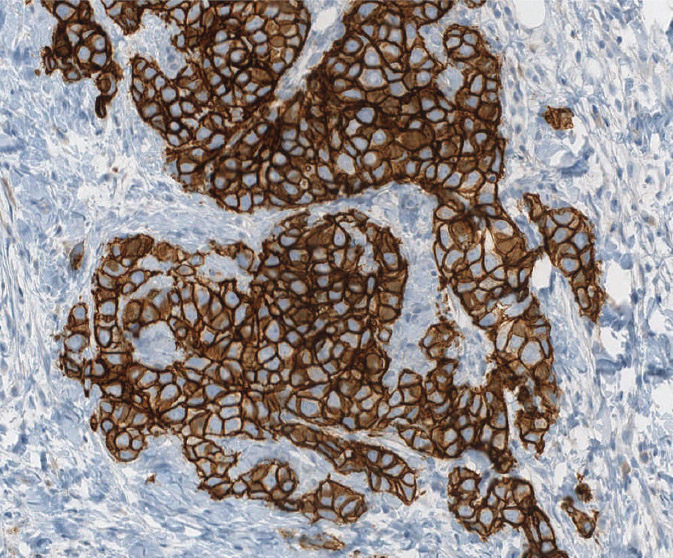
Figure 5.8 Strongly positive (3+) HER2/neu immunostain.
HER2 Testing Guidelines (ASCO/CAP 2013) (48)
HER2 TESTING BY IHC
• Score 0 (negative)—no staining or only incomplete, faint membrane staining in ≤10% of tumor cells
• Score 1+ (negative)—incomplete, faint membrane staining in >10% of tumor cells
• Score 2+ (equivocal)—incomplete and/or weak to moderate circumferential membrane staining occurs in >10% of tumor cells or complete, intense, circumferential in ≤10% of tumor cells
• Score 3+ (positive) is to be reported when complete, intense, circumferential staining is demonstrated in >10% of tumor cells
FLUORESCENCE IN SITU HYBRIDIZATION (FISH), HER POSITIVITY
• Single-probe average HER2 copy number ≥6.0 signals/cell
• Dual-probe HER2/CEP17 ratio ≥2.0 with an average HER2 copy number ≥4.0 signals per cell
• Dual-probe HER2/CEP17 ratio ≥2.0 with an average HER2 copy number <4.0 signals/cell
• Dual-probe HER2/CEP17 ratio <2.0 with an average HER2 copy number ≥6.0 signals/cell
Ki-67 is a nuclear protein expressed in dividing cells; it is a reliable marker of cell proliferation and has been shown to correlate with tumor grade. Moreover, studies have shown that the Ki-67 index is an independent prognostic factor, correlating significantly with disease-free and overall survival in BC patients (49). In 2009, the Saint Gallen consensus conference determined cutoffs for the Ki-67 index. It was recommended that a Ki-67 proliferative index of less than 15% be considered low/favorable, a Ki-67 proliferative index of 16% to 30% be deemed “not useful for decision,” and an index greater than 30% be considered unfavorable.
Prognostic and Predictive Multigene Tests
In the recent past, tumor biomarker tests have pursued a single target. However, the past decade has seen huge advances in –omics-based technology, in which numerous analytes (eg, expression of several genes) are measured. Commercially available multigene tests and their clinical validation trials are discussed later in the chapter (Table 5.8). These multigene tests have been used for prognostics and prediction of benefit from chemotherapy and extended hormone therapy.
Other Cancers Presenting in the Breast
While metastasis of extramammary malignancies to the breast accounts for a rare minority of breast lesions (50,51), a wide range of primary tumor sites and histological types giving rise to secondary breast lesions have been reported in both women and men. Lymphomas are the most frequent culprits, with melanoma; carcinomas of the lung, gastrointestinal tract, genitourinary tract, and gynecological tract; and sarcomas of the uterus also featuring prominently (52–56).
To distinguish primary breast tumors from extramammary metastases, the constellation of gross examination, cellular morphology, tissue architecture, IHC staining, patient history, and clinical presentation must be considered. Typical metastatic lesions are solitary, round, well-circumscribed nodules in a background of fibrotic breast tissue, with absence of in situ mammary carcinoma. In contrast, primary breast tumors often have spiculated borders that infiltrate the breast parenchyma and feature elastosis and calcification (53,57). Microscopically, features characteristic of specific tissue origin are often observed within metastases and certain pathognomonic clues may be adequate in some cases to render a diagnosis (58,59). Comparison with pathological specimens of previously diagnosed primary tumors, when available, can be particularly helpful.
Immunophenotype of a breast lesion, determined by a panel of carefully chosen antibody markers, plays a supporting role in augmenting or refuting the identification of a metastasis to the breast. For poorly differentiated metastatic tumors in patients lacking a diagnosis of a primary malignancy or metastases that mimic primary BCs or benign breast lesions, immunochemistry may form the cornerstone for diagnosis (60). For example, high-grade serous carcinoma of the ovary metastatic to the breast is frequently misdiagnosed as a primary breast carcinoma due to overlapping morphologic and IHC features (53). While both malignancies may have an estrogen receptor +/progesterone receptor +/cytokeratin 7 +/cytokeratin 20– expression profile, PAX8 and Wilms tumor (WT-1) can be used to distinguish between breast and ovarian origin. Ovarian tumors tend to be PAX8+/WT-1+, while breast tumors are typically PAX8−/WT-1− (57).
SURGERY
Historical Perspective
Described by William Stewart Halsted in 1894, the radical mastectomy was the standard of care for operable BC throughout most of the 20th century (61). The procedure involved removal of the entire breast, ipsilateral pectoralis muscles, and axillary lymph nodes and was associated with significant morbidity (61). Over the past several decades, a number of key clinical trials have prompted major advances in the surgical management of early-stage invasive BC. Radical mastectomy has been replaced by modified radical mastectomy (sparing the pectoralis muscles as well as decreasing the extent of the axillary dissection with removal of only level 1 and 2 lymph nodes) and then by BCS toward the end of the 20th century as several clinical trials have shown that less extensive surgical procedures provide equivalent oncologic outcomes and result in lower morbidity (62).
Management of the Breast
Surgery for BC consists of removal of the tumor by either BCS or mastectomy and simultaneous evaluation of the regional lymph nodes by sentinel lymph node biopsy (SLNB) or axillary lymph node dissection (ALND).
Breast Conserving Surgery
BCS, also referred to as a lumpectomy, partial mastectomy, segmentectomy, quadrantectomy, and wide local excision, involves removal of the primary tumor with a surrounding rim of normal breast tissue while preserving the remainder of the breast. Since the majority of breast tissue is left intact, the incision is closed primarily and no reconstruction is required. This approach is routinely combined with adjuvant radiation therapy (RT) to reduce the incidence of local recurrence (LR), which translates into a survival advantage described further in the radiation section. Table 5.4 summarizes the prospective trials that compared BCS with radiation to mastectomy.
MARGINS
There has been significant controversy over the years regarding adequate margin width in patients undergoing BCS for invasive BC. The most recent data demonstrate no significant difference in LR rates when negative margins, defined as “no tumor on ink,” are achieved in patients undergoing BCS and whole breast irradiation (WBI) and who receive appropriate adjuvant systemic therapy (69,70). However, patients with multifocal (MF) close margins with “no tumor on ink” may still need to be considered for reexcision. This may be particularly important in patients with an extensive intraductal component. Therefore, it is essential to review each patient’s pathology individually to ensure that adequate margins are obtained.
 We currently consider “no tumor on ink” as an adequate margin in patients with invasive BC. In patients with a combination of invasive BC and DCIS, while “no tumor on ink” is considered an adequate margin, reexcision may be necessary depending on the extent of DCIS.
We currently consider “no tumor on ink” as an adequate margin in patients with invasive BC. In patients with a combination of invasive BC and DCIS, while “no tumor on ink” is considered an adequate margin, reexcision may be necessary depending on the extent of DCIS.
BCS ELIGIBILITY
Absolute contraindications include diffuse suspicious or malignant appearing calcifications on imaging, persistently positive margins after multiple excisions, multicentric (MC) disease (two foci of disease in different quadrants) that cannot be removed with a single excision, early pregnancy, and prior thoracic radiation.
Relative contraindications include large tumors that will lead to poor cosmesis, very large breast size, patients who are not candidates for postoperative RT and multifocal (MF) disease (two foci in the same quadrant). For patients with large tumors who desire BCS, neoadjuvant systemic therapy may be considered to reduce tumor size and increase eligibility for BCS.
Although the use of BCS in MF or MC disease is controversial, a study from the European Institute of Oncology in Milan, in which 421 patients with MF cancer and 55 with MC cancer were treated with BCS, demonstrated a 5-year cumulative incidence of LR of 5.1% (71). For patients with one of these relative contraindications, a full disclosure of risks and benefits of each option should be discussed.
Mastectomy
Mastectomy removes the entire breast. This procedure may be considered in patients with all stages of BC. Mastectomy is often combined with breast reconstruction in patients who are candidates.
TYPES OF MASTECTOMY
Simple mastectomy (total mastectomy): complete removal of the breast including the nipple–areolar complex (NAC).
Modified radical mastectomy: removal of the breast, NAC, and level 1 and 2 axillary lymph nodes.
Skin-sparing mastectomy: removal of all breast tissue and the NAC with preservation of the skin envelope of the breast.
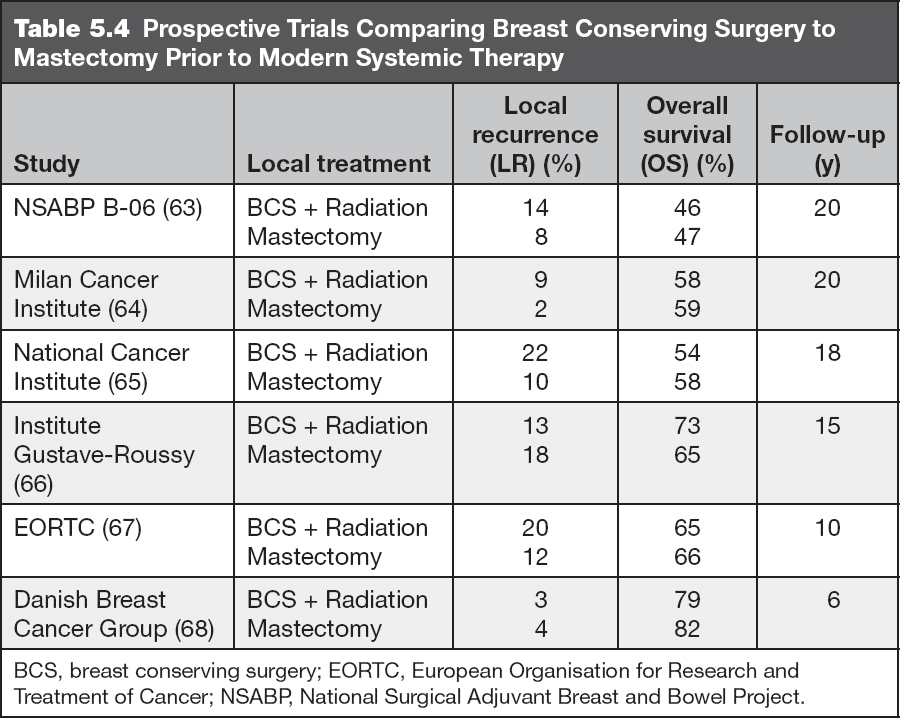
Nipple-sparing mastectomy (NSM): removal of all breast tissue while preserving the entire skin envelope of the breast including the NAC. This approach is often used for prophylactic mastectomy in high-risk patients or for patients with small BCs located away from the NAC (Figure 5.9).
Skin-sparing mastectomy outcomes. The majority of patients in studies of skin-sparing mastectomy had early-stage disease (stage 0–II) and the reported LR rates ranged from 1.9% to 7% with follow-up times of 49 to 118 months (72–77). Patients are generally considered eligible for skin-sparing mastectomy if they have early-stage BC, stage 0–II. While this procedure has been examined in patients with more advanced BC (stage IIB and III), the studies are small and the oncologic safety cannot be confirmed (78–80).
NSM outcomes. Factors associated with NAC involvement include larger tumors, smaller distance between the tumor and NAC, MC disease, lymph node involvement, higher tumor grade, and HER2 amplification. NSM was initially utilized in high-risk patients undergoing prophylactic mastectomy for risk reduction. However, the procedure has since been expanded to patients with small, unifocal tumors (<2–3 cm) that are at least 1 to 2 cm from the NAC (81–83). In studies that have evaluated the procedure in patients with BC, NAC involvement was seen in 8% to 33% of cases (84). Relative contraindications to the procedure include significant ptosis in the breasts, large breasts, smoking, diabetes, and obesity (84). Small studies that examined oncologic outcomes in patients undergoing NSM showed LR rates of 2% to 3% at follow-up times of 28 to 60 months (81–83).
Figure 5.9 Bilateral nipple sparing mastectomy with implant reconstruction.
 Currently, we consider NSM for patients with unifocal BCs that are <3 cm in size and at least 1 to 2 cm away from the NAC.
Currently, we consider NSM for patients with unifocal BCs that are <3 cm in size and at least 1 to 2 cm away from the NAC.
Surgical Staging of Lymphatics
Following the trend toward less invasive and morbid procedures, surgical management of the axilla has moved away from the extensive axillary dissections to targeted removal of axillary lymph nodes through lymphatic mapping and SLNB.
Surgical Options
SLNB is a less morbid alternative to ALND for staging of the regional lymph nodes in patients with BC with clinically negative lymph nodes (85,86). This technique involves the targeted identification and removal of the first draining lymph nodes of the breast, which are located in the axilla in the majority of cases. The mapping procedure uses a radiolabeled tracer and/or blue dye, which is injected into the breast tissue (either intradermal, peritumoral, periareolar, or subareolar), and then travels through the lymphatics and accumulates in the sentinel node(s) (85–89). The sentinel lymph nodes (SLNs) may be detected at the time of surgery by visualization of blue dye or by radioactivity detection using a gamma probe. The combination of dye and radiotracer results in an SLN detection rate of over 95% with false-negative rates (FNRs) reported between 0% and 10% (88–90). After a negative SLNB without ALND, axillary recurrence rates are extremely low (91).
 We recommend SLNB in patients with early-stage, clinically nodenegative invasive BC, and in patients with DCIS undergoing mastectomy.
We recommend SLNB in patients with early-stage, clinically nodenegative invasive BC, and in patients with DCIS undergoing mastectomy.
Patient Eligibility for SLNB
Clinical situations in which there is insufficient data to support SLNB include patients with T3/T4 BC, locally advanced disease, inflammatory BC, DCIS when BCS is planned, and pregnancy. We typically consider SLNB in patients with T3 tumors. In pregnant patients with early-stage BC, we consider SLNB with the use of radiolabeled tracer alone. In addition, in patients with DCIS, we perform SLNB in patients with a large area of involvement (>5 cm), a palpable mass, imaging findings concerning for an invasive BC, and in cases where SLNB may not be successful after BCS due to location of disease.
AXILLARY LYMPH NODE DISSECTION
This procedure is utilized for patients with clinically positive lymph nodes or in patients with multiple positive SLNs. It involves removal of the level 1 and 2 axillary lymph nodes. Level 3 lymph nodes are only removed when they are grossly positive, although this may significantly increase morbidity due to an increase in lymphedema. This procedure provides regional disease control in patients.
Management of Clinically Negative Axillary Lymph Nodes
The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial examined and confirmed the efficacy of SLNB in patients with early-stage BC. This study included patients with clinically negative lymph nodes who were randomized to either SLNB with ALND or SLNB alone (with ALND only if the SLN was positive). In patients who had pathologically negative SLNs, outcomes were statistically equivalent between the two groups, including 8-year OS (91.8% SLNB + ALND vs. 90.3% SLNB alone; P = .12), disease-free survival (DFS) (82.4% vs. 81.5%; P = .54) and regional disease control (0.4% vs. 0.7%; P = .22) (91).
 For patients with clinically negative axillary nodes and pathologically negative SLNs, no further axillary surgery is needed.
For patients with clinically negative axillary nodes and pathologically negative SLNs, no further axillary surgery is needed.
The American College of Surgeons Oncology Group (ACOSOG) Z0011 trial examined the need for ALND in patients with early-stage BC and one or two positive SLNs who were planning to undergo BCS followed by systemic therapy and WBI (92,93). Patients were randomized to either ALND or SLNB alone. The trial accrued slowly and closed early, but demonstrated no significant difference between ALND and SLNB at 5 years in terms of LR (3.1% vs. 1.6% SLNB; P = .11), regional recurrence (0.5% vs. 0.9%; P = .45), DFS (82.2% vs. 83.9%; P = .14), and OS (91.8% vs. 92.5%; P = .25) (92,93). For patients with clinically negative axillary nodes with one or two pathologically positive SLNs, no further axillary dissection is necessary as long as whole breast radiation and appropriate adjuvant systemic therapy is planned.
 The 2014 ASCO guidelines now recognize that patients with one or two positive SLNs who undergo BCS and receive appropriate adjuvant systemic therapy and WBI do not require ALND.
The 2014 ASCO guidelines now recognize that patients with one or two positive SLNs who undergo BCS and receive appropriate adjuvant systemic therapy and WBI do not require ALND.
One caveat to this study was that the volume irradiated was not standardized, as standard tangential radiation does not cover the entire volume that would be encompassed in a level 1/2 axillary dissection, such that some patients received larger radiation volumes to the entire axilla (ie, high tangents in 50% of patients on Z0011) or received radiation to the entire axilla and supraclavicular fossa (19% of patients on Z0011).
The IBCSG-23-01 trial also compared SLNB and ALND to SLNB alone in patients with positive SLNs and included patients with T1/2 N0 disease and the presence of one or more SLNs with micrometastases (<2 mm) (94). Unlike Z0011, patients undergoing mastectomy were also included and comprised about 10% of the patient population and did not undergo radiotherapy. The 5-year DFS was similar between those who underwent ALND (84.4%) and SLNB alone (87.8%), P = .16 (94).
The AMAROS trial evaluated radiation targeting the axilla (levels 1–3) and supraclavicular fossa as an alternative to ALND in patients with clinical T1/2 N0 BC with positive SLNs (95). Patients with at least one positive SLN were randomized to external radiation therapy (XRT) or ALND. The 5-year results showed similar axillary recurrence rates in the two groups (ALND 0.54% vs. XRT 1.03%) (95). The lymphedema rates were lower in those patients undergoing axillary XRT, with clinical signs of lymphedema in ALND 23% versus XRT 11% (P < .0001) and with an arm circumference increase of >10% in ALND 13% versus XRT 5% (P < .0009) (95).
Clinical Node-Negative, SLN Positive, Candidates for ALND
Completion ALND (levels 1 and 2) may be offered to some patients with positive SLNs. This includes patients who do not meet criteria for ACOSOG Z0011 or the IBCSG-23-01 trial.
• Patients who have more than two positive SLNs.
• Patients undergoing mastectomy who have macrometastatic disease in SLNs and will not receive adjuvant XRT.
• Patients who receive neoadjuvant therapy.
• Patients who cannot receive recommended adjuvant systemic therapy and RT.
Management of Clinically Positive Axillary Nodes
 We recommend axillary ultrasound (US) and FNA or core biopsy of clinically palpable or mammographically/US/MRI suspicious lymph nodes to confirm involvement.
We recommend axillary ultrasound (US) and FNA or core biopsy of clinically palpable or mammographically/US/MRI suspicious lymph nodes to confirm involvement.
If biopsy is negative, SLNB should be performed at surgery for appropriate staging.
If biopsy is positive, ALND at surgery or neoadjuvant systemic therapy may be considered. In patients who will receive neoadjuvant systemic therapy we recommend core biopsy and clip placement so that the biopsied lymph node may be localized and removed at the time of surgery.
Conclusions
The surgical management of early-stage invasive BC has changed significantly over the past several decades. Based on the outcomes from a number of pivotal clinical trials, clinicians are able to offer increasingly less extensive surgical procedures while maintaining oncologic outcomes. While the surgical management of primary tumors and lymph nodes is constantly evolving, the overall goal of improving patient outcomes through multimodality therapy remains the same.
RADIATION THERAPY IN THE ADJUVANT SETTING FOLLOWING SURGERY
RT complements surgery by reducing the incidence of local and regional failures. In the modern era of systemic therapy, these reductions in local–regional disease have had an impact on reducing distant metastases, as certain regional disease can act as a harbinger of distant disease, and have also had an impact on survival (96). In addition, the delivery of radiation has improved substantially with novel technologies that reduce the morbidity and mortality of therapy, causing an overall improvement in quality of life for patients with BC.
RADIATION THERAPY AS AN ADJUVANT TO BCS FOR EARLY-STAGE BC
Comparison of BCS to Modified Radical Mastectomy (MRM) for Women With Tumors <5 cm in Size
As reviewed in Table 5.4, six randomized trials were performed several decades ago comparing BCS and whole breast radiation versus mastectomy. The results of the studies demonstrated similar local control, DFS, and OS between the two approaches. Due to psychological and quality of life benefits of BCS, the National Cancer Institute (NCI)/National Institutes of Health (NIH) Consensus described BCS as the “preferable” strategy in managing early-stage BC in eligible and appropriate patients (97).
BCS Without Radiation in an Unselected Population
In an attempt to improve quality of life further, many trials have tried to forgo radiation completely following BCS. In the unselected population, the absence of radiation was associated with recurrence and detriments in survival as described in the following.
NSABP B-06 accrued 1,851 women with primary tumors <4 cm who were randomized to total mastectomy versus wide local excision and radiation versus wide local excision alone (98). While there was no OS benefit with the addition of radiation, there was substantial (39.2% vs. 14.3%; P < .001) reduction in ipsilateral BC recurrence. There were, however, suggestions in this trial that adjuvant radiotherapy may affect survival in that BC deaths were reduced marginally (hazard ratio [HR] 0.82; P = .04) with the addition of radiation to lumpectomy. This effect was somewhat offset by competing all-cause deaths, later attributed to the negative cardiac effects of radiotherapy. However, it should be noted that WBI in this trial was delivered with what would now be considered antiquated techniques and current radiation volumes to the heart are significantly lower.
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) subsequently published an overview encompassing 78 randomized trials, 42,000 women, and 15-year results (99). With the increased power from the large sample size, an OS benefit of 5.3% (P = .005) was realized with the addition of radiotherapy to lumpectomy. For every four local, in-breast recurrences prevented with radiotherapy, one woman was saved from death from BC. Importantly, this analysis demonstrated that interventions affecting a 10% or more improvement in local control at the primary site at 5 years resulted in a reduction in BC mortality at 15 years.
BCS Without Radiation in a Selected Population
EBCTCG asserts that interventions with minimal local control benefit fail to yield a survival benefit (100). A meta-analysis including 17 randomized trials, 10,801 women, and 15 years of follow-up sought to identify individual prognostic factors that could identify patients who benefit minimally from adjuvant radiotherapy. Despite examining age, tumor size, tumor grade, ER status, additional therapy, and surgical procedure extent, no subgroup was identified in which it was completely safe to omit radiotherapy without a decrease in local control.
NSABP B-21: Investigators then postulated that hormonal therapy might replace RT in an unselected population and randomized 1,009 women with tumors less than 1 cm in diameter and node-negative disease to one of three arms following lumpectomy—tamoxifen alone, RT and placebo, or RT and tamoxifen (101). Eight-year follow-up showed no difference in distant failure or OS between the three groups. RT reduced recurrences more than tamoxifen alone (16.5% vs. 9.3% at 8 years), but the best in-breast control was seen with the combination of RT and tamoxifen (2.8% at 8 years).
The Cancer and Leukemia Group B (CALGB) 9343 trial was more selective and enrolled 636 low-risk patients who were 70 years of age or older with ER+ (99% of cases) disease with tumors that were <2 cm (98% of cases) in maximum dimension who were treated with lumpectomy and tamoxifen. The randomization was between radiation and no radiation with the primary end point being a difference in local control (102). The 12-year update showed a significant improvement in local control (10% without radiation vs. 2% with radiation) (103) without a difference in OS. This trial is often mistakenly identified as demonstrating a lack of benefit of radiotherapy. Nonetheless, this treatment approach has emerged as a reasonable, but not necessarily preferred, option for women over 70 with the most favorable BC characteristics.
The PRIME II study has served to affirm the efficacy of this strategy despite relatively short follow-up (104). With a similar randomization of women 65 and older, ipsilateral BC recurrence in the 5-year report was 1.3% versus 4.1% with and without radiotherapy, respectively, similar to the 5-year results of the CALGB trial.
These findings are the best to date in identifying a group that may be able to forgo adjuvant radiation without detriment to survival. However, it should be noted that these trials consistently demonstrate a benefit in local–regional control with the addition of radiotherapy, even in highly selected populations. For women older than 70 electing to forgo radiation based on the previous data, consideration of a “trial period” for women electing to proceed with adjuvant hormone therapy alone can be employed. If either tolerance or compliance is poor at 3 to 4 months following surgery, radiotherapy could be employed at this juncture, either in place of or along with continued medical therapy.
 For patients who refuse or cannot tolerate hormonal therapy or have a life expectancy of 10 years or more, radiotherapy is recommended.
For patients who refuse or cannot tolerate hormonal therapy or have a life expectancy of 10 years or more, radiotherapy is recommended.
Techniques of Radiation
Deep inspiratory breath-hold requires the patient to inspire deeply and hold her breath while individual fields of radiotherapy are administered. This technique moves the heart medially and inferiorly, substantially reducing the heart dose without sacrificing breast tissue coverage (see Figures 5.10A and 5.10B). For many patients, this is a relatively simple procedure with reliable reproducibility when the stability of the breath can be monitored during radiation (105).
Prone breast radiation allows for the breast to fall away from the chest wall. In women with larger breasts, this approach can be particularly advantageous in reducing redundant breast folds, which can lead to more substantial acute skin toxicity, as well as improving dose homogeneity, which can reduce both acute and long-term side effects (see Figure 5.11). This technique can also aid in reducing the dose to the heart and lungs (106,107). However, this technique is not utilized when treatment of the regional nodes is necessary. There is also concern that the chest wall can be underdosed with this approach and should be used with caution for patients in whom chest radiation may be of benefit (ie, triple negative BC).
Figure 5.10 (A) Techniques of radiation—free breathing. (B) Techniques of radiation—deep inspiration breath-hold. Standard breast positioning; redundant breast folds can cause increased skin toxicity.
Figure 5.11 Prone breast radiation allows the breast to fall away from the chest wall.
Intensity-modulated radiation therapy (IMRT) is a sophisticated method of radiation delivery used for BC in two situations. In early-stage disease, it is used to make the dose delivery more homogeneous, which has been shown in randomized clinical trials to reduce acute grade 2–3 toxicity compared to conventional RT (108,109). The second approach is used in more advanced disease to conform the high doses of radiation around nearby critical structures. The largest experience in early disease was published from Fox Chase Cancer Center, which demonstrated the superiority of using breast IMRT compared to conventional radiation (110). The series included 804 consecutive women with early-stage BC who were treated with BCS and radiation from 2001–2006, where 399 had received photon IMRT. The maximum toxicity (grade 2 and higher) was significantly reduced using IMRT, 75% for conventional radiotherapy and 52% for IMRT (P < .0001). In addition, the duration (percentage of treatment weeks) patients spent experiencing grade 2 and higher dermatitis was substantially reduced from 71% for conventional radiation versus 18% for those who received IMRT (P < .0001). Two smaller retrospective series demonstrated similar benefits with decreased acute skin toxicity grade 2 or 3 (39% vs. 52%; P = 0.047) (111) and reduced grade 2 or greater breast edema (0% vs. 36%; P < .001) (112). There is also improvement in late effects with reduction in the risk of developing chronic grade 2 or greater breast edema (3% vs. 30%; P = .007) (112) and improved cosmesis (113).
In a randomized phase III prospective trial from Canada of 358 patients, conventional radiation was compared to IMRT. It was found that IMRT was associated with improved dose homogeneity and reduced moist desquamation (31% vs. 48%; P = .0019) (109). This translated into a quality of life benefit for those patients who experienced moist desquamation. A randomized trial from the United Kingdom compared standard radiotherapy to IMRT in patients with early-stage BC and 240 of 306 patients were able to be evaluated by photographs for change in breast appearance (108). There was a negative change in breast appearance in 58% of patients randomized to conventional treatment compared to 40% randomized to IMRT. Two-dimensional (2D) RT was 1.7 times more likely to have a change in breast appearance than IMRT (P = .008).
WHOLE BREAST IRRADIATION—FRACTIONATION
Standard adjuvant radiotherapy for BC has traditionally incorporated 5 to 5.5 weeks of WBI followed by a “boost” delivered over 1 to 1.5 additional weeks to the lumpectomy cavity. The whole breast is targeted to ensure full coverage of the postsurgical cavity as well as occult MC disease within the remainder of the breast (114). Results from randomized trials have suggested that radiotherapy may be hypofractionated in early-stage BC—delivering a radiobiologically similar dose over 3 to 4 weeks (115–121).
The UK Standardization of Breast Radiotherapy (START) A and START B trials were intended to elucidate inherited radiobiologic differences between normal breast tissue and BC such that similar cell kill could be achieved through a shorter course of RT without altering acute or late effects. Multiple randomized phase III trials have tested this concept in comparison to conventional fractionation.
START A included 2,236 women with early-stage BC treated with partial mastectomy and then split them into three treatment groups to receive either 50 Gy in 25 fractions (2 Gy per fraction), 41.6 Gy in 13 fractions (3.2 Gy per fraction), or 39 Gy in 13 fractions (3 Gy per fraction) (115). At 5-year follow-up, all three arms had similar local control. There was a slightly higher rate of failures in the 39 Gy arm, although this was not statistically significant.
START B randomized 2,215 women to 50 Gy in 25 fractions or 40 Gy in 15 fractions (2.67 Gy per fraction) given over 3 weeks (116). Again, at 5 years, local–regional relapse was not significantly different between the 50 Gy (2.2%) and 40 Gy (3.3%) cohorts. At 10-year follow-up, these results have held, further solidifying shortened regimens as viable options in this disease (122). Concerns regarding the potential for increased late toxicity with higher doses per fraction have proven to be unfounded at 10 years of follow-up.
The Ontario Clinical Oncology Group (OCOG) performed a randomized trial in the mid-1990s with similar structure and intent to shorten duration (118,119,121). This effort enrolled 1,234 women with T1 or T2, node-negative BC treated with BCS who were randomized to whole breast RT, either 50 Gy in 25 fractions or 42.5 Gy in 16 fractions. The 10-year results showed comparable rates of local control (6.7% vs. 6.2%) with nearly identical rates of good to excellent cosmesis (71.3% vs. 69.8%). Two recent reports suggesting improvements in acute toxicity with hypofractionation should further bolster this effect (123,124).



